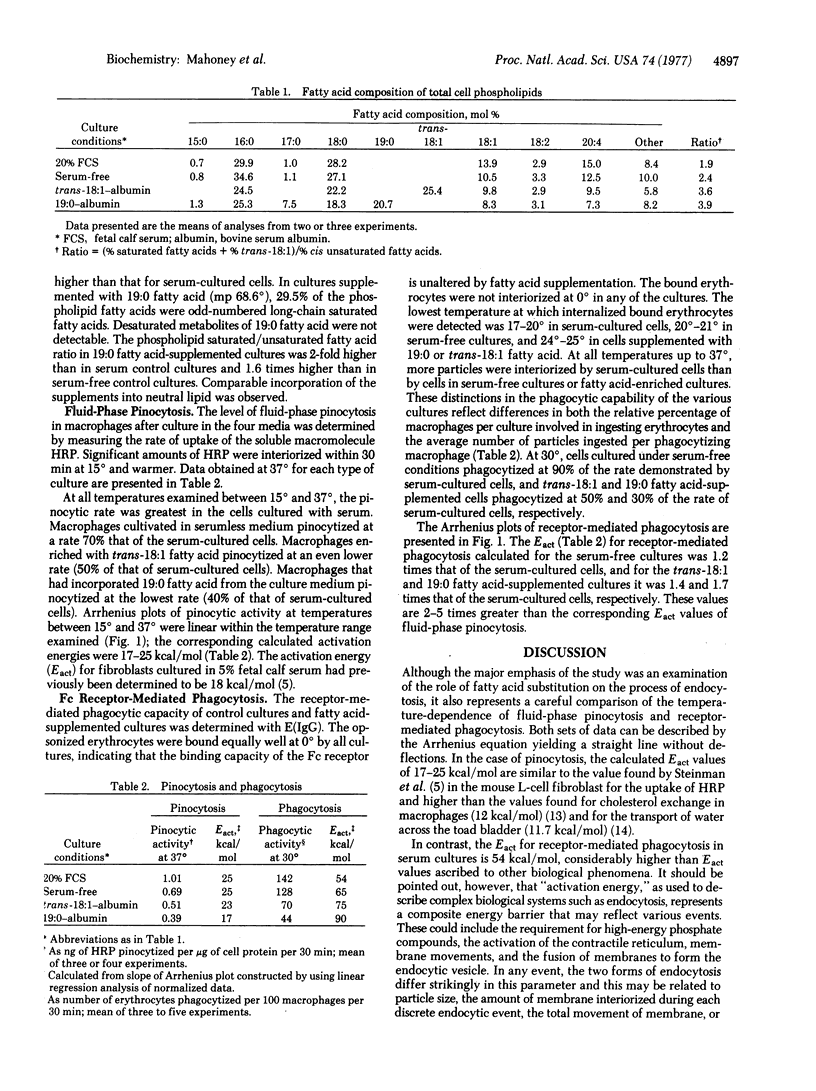
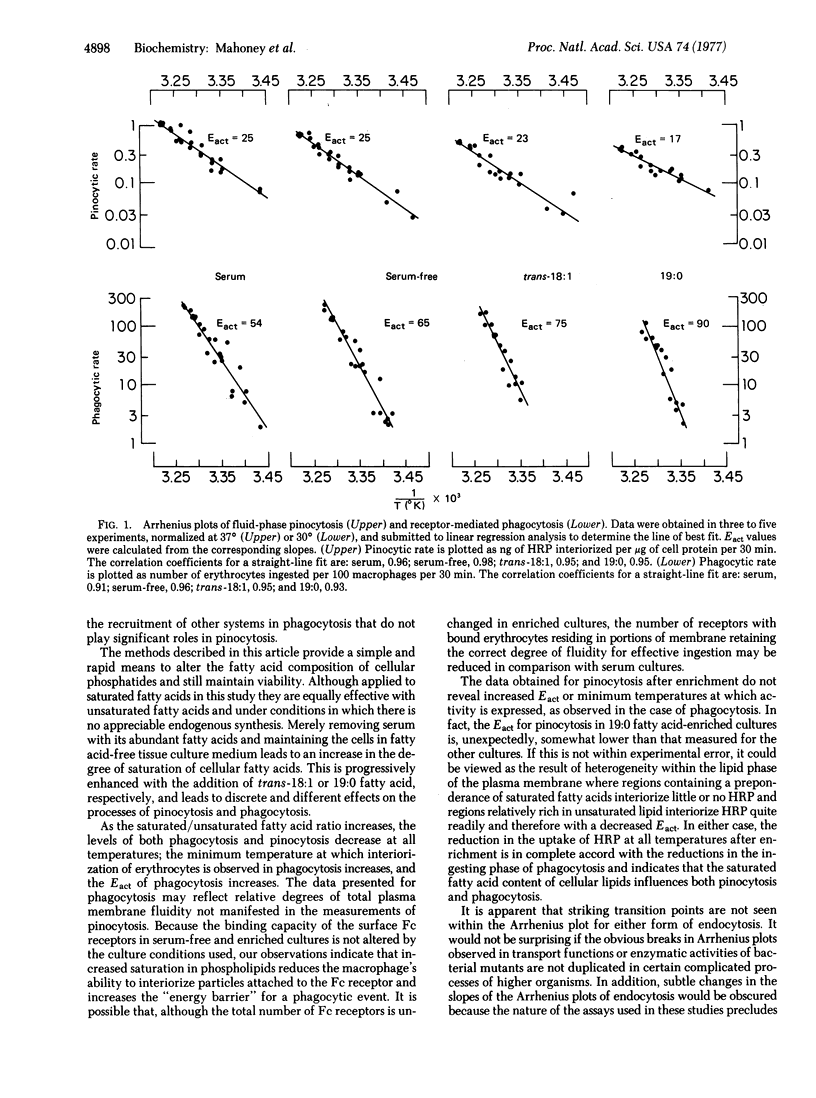
Abstract
Mouse peritoneal macrophages incubated in serumless medium containing a 19:0 or trans-18:1 fatty acid complexed to bovine serum albumin incorporate the exogenous fatty acid supplement into cellular phospholipids. Within 8 hr, 25% of the total phospholipid fatty acids are derived from the supplement, with cell viability remaining greater than 95%. The incorporation of either of these supplements increases the saturated/unsaturated fatty acid ratio in the phospholipids 2-fold over that of cells cultured in serum and effects striking changes in endocytic activities. The levels of both fluid-phase pinocytosis and receptor-mediated phagocytosis are decreased at all temperatures examined between 15 degrees and 37 degrees. The increased degree of saturation of cell phospholipids correlates with decreased endocytic rates for both processes and with increased activation energies (Eact) for phagocytosis. The Eact values for phagocytosis, which range from 54 to 90 kcal/mol, depend on the supplementation conditions used. Although the levels of pinocytosis are depressed, the Eact values for pinocytosis (17--25 kcal/mol) are not strikingly affected by saturated fatty acid enrichment. These observations suggest that the degree of lipid fluidity of macrophage membranes influences both phagocytosis and pinocytosis in macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. A., Glaser M., Bayer W. H., Vagelos P. R. Alteration of fatty acid composition of LM cells by lipid supplementation and temperature. Biochemistry. 1975 Jan 14;14(1):146–151. doi: 10.1021/bi00672a025. [DOI] [PubMed] [Google Scholar]
- Gordon S. Macrophage neutral proteinases and chronic inflammation. Ann N Y Acad Sci. 1976;278:176–189. doi: 10.1111/j.1749-6632.1976.tb47028.x. [DOI] [PubMed] [Google Scholar]
- Hays R. M., Franki N., Soberman R. Activation energy for water diffusion across the toad bladder: evidence against the pore enlargement hypothesis. J Clin Invest. 1971 May;50(5):1016–1018. doi: 10.1172/JCI106572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. F., Hatten M. E., Burger M. M. Membrane fatty acid replacements and their effect on growth and lectin-induced agglutinability. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3115–3119. doi: 10.1073/pnas.71.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse H. G., Williams R. E., Wisnieski B., Fox C. F. Alterations of characteristic temperatures for lectin interactions in LM cells with altered lipid composition. Biochem Biophys Res Commun. 1974 May 7;58(1):222–228. doi: 10.1016/0006-291x(74)90915-2. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Rottem S., Gallily R. Motion of spin-labeled fatty acids in murine macrophages. Relation to cellular phagocytic activity. Biochim Biophys Acta. 1976 Mar 19;426(3):499–512. doi: 10.1016/0005-2736(76)90394-1. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Cholesterol metabolism in the macrophage. I. The regulation of cholesterol exchange. J Exp Med. 1971 Dec 1;134(6):1545–1569. doi: 10.1084/jem.134.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. E., Wisnieski B. J., Rittenhouse H. G., Fox C. F. Utilization of fatty acid supplements by cultured animal cells. Biochemistry. 1974 Apr 23;13(9):1969–1977. doi: 10.1021/bi00706a029. [DOI] [PubMed] [Google Scholar]


