Abstract
Objective
To determine if repetitive sphenopalatine ganglion (SPG) blocks with 0.5% bupivacaine delivered through the Tx360® are superior in reducing pain associated with chronic migraine (CM) compared with saline.
Background
The SPG is a small concentrated structure of neuronal tissue that resides within the pterygopalatine fossa (PPF) in close proximity to the sphenopalatine foramen and is innervated by the maxillary division of the trigeminal nerve. From an anatomical and physiological perspective, SPG blockade may be an effective acute and preventative treatment for CM.
Method
This was a double-blind, parallel-arm, placebo-controlled, randomized pilot study using a novel intervention for acute treatment in CM. Up to 41 subjects could be enrolled at 2 headache specialty clinics in the US. Eligible subjects were between 18 and 80 years of age and had a history of CM defined by the second edition of the International Classification of Headache Disorders appendix definition. They were allowed a stable dose of migraine preventive medications that was maintained throughout the study. Following a 28-day baseline period, subjects were randomized by computer-generated lists of 2:1 to receive 0.5% bupivacaine or saline, respectively. The primary end-point was to compare numeric rating scale scores at pretreatment baseline vs 15 minutes, 30 minutes, and 24 hours postprocedure for all 12 treatments.
SPG blockade was accomplished with the Tx360®, which allows a small flexible soft plastic tube that is advanced below the middle turbinate just past the pterygopalatine fossa into the intranasal space. A 0.3 cc of anesthetic or saline was injected into the mucosa covering the SPG. The procedure is performed similarly in each nostril. The active phase of the study consisted of a series of 12 SPG blocks with 0.3 cc of 0.5% bupivacaine or saline provided 2 times per week for 6 weeks. Subjects were re-evaluated at 1 and 6 months postfinal procedure.
Results
The final dataset included 38 subjects, 26 in the bupivacaine group and 12 in the saline group. A repeated measures analysis of variance showed that subjects receiving treatment with bupivacaine experienced a significant reduction in the numeric rating scale scores compared with those receiving saline at baseline (M = 3.78 vs M = 3.18, P = .10), 15 minutes (M = 3.51 vs M = 2.53, P < .001), 30 minutes (M = 3.45 vs M = 2.41, P < .001), and 24 hours after treatment (M = 4.20 vs M = 2.85, P < .001), respectively. Headache Impact Test-6 scores were statistically significantly decreased in subjects receiving treatments with bupivacaine from before treatment to the final treatment (Mdiff = −4.52, P = .005), whereas no significant change was seen in the saline group (Mdiff = −1.50, P = .13).
Conclusion
SPG blockade with bupivacaine delivered repetitively for 6 weeks with the Tx360® device demonstrates promise as an acute treatment of headache in some subjects with CM. Statistically significant headache relief is noted at 15 and 30 minutes and sustained at 24 hours for SPG blockade with bupivacaine vs saline. The Tx360® device was simple to use and not associated with any significant or lasting adverse events. Further research on sphenopalatine ganglion blockade is warranted.
Keywords: chronic migraine, Tx360®, sphenopalatine ganglion block, preventive treatment, acute treatment
Migraine is a highly prevalent medical disorder considered to be a leading cause of worldwide disability.1 Historically, migraine has been viewed as a self-limited episodic pain disorder, but there is growing acceptance in the medical community of a disease model whereby chronic migraine (CM) is considered the end-stage consequence of uncontrolled or poorly controlled episodic migraine (EM).2,3 In fact, the International Headache Society (IHS) has stated that CM should be considered a complication of EM.4
CM is a relatively new diagnosis first established in the second edition of the International Classification of Headache Disorders (ICHD-II) in 2004. Diagnostic criteria were redefined in 2006 as an appendix addition to ICHD-II5 and further refined in the ICHD-III beta criteria published in 2013.6 However, the 2006 appendix criteria used in this study are very similar to the ICHD-III beta criteria. Basically, these criteria define CM as 15+ days/month of headache lasting >4 hours for at least 3 months with at least 8 days of headache meeting diagnostic criteria for EM.7 In CM, symptom intensity, duration, and attack disability are increased relative to EM. CM is also associated with a significantly greater degree of healthcare utilization and comorbidity.8
A consequence of migraine being separated into episodic and chronic diagnoses is that few scientifically validated clinical studies that specifically address the efficacy and safety of acute treatment in the CM population exist. Although it is widely assumed that acute interventions approved for EM are effective in CM, the fact remains that the CM patient population was excluded from almost all regulatory trials of acute migraine medications. Thus, no large clinical trials of acute medications have been conducted specifically in CM populations. Complicating matters are the constructs of medication overuse (MO) and medication overuse headache (MOH), suggesting that overuse of acute medications is an important catalyst in chronification and maintenance of CM. This underscores a significant difference in the risk for acute medications when used indiscriminately in patients with episodic vs CM. Clearly, there is a need to study the use of acute treatment and novel interventions in CM to define effective treatment strategies for this important patient population.
The Sphenopalatine Ganglion (SPG)
The SPG is a small concentrated structure containing the largest group of neurons outside the brain. It resides within the pterygopalatine fossa (PPF) in close proximity to the sphenopalatine foramen. It is located at the posterior attachment of the middle turbinate above the ethmoidal crest. The SPG is innervated by the maxillary division of the trigeminal nerve. It has a sensory, parasympathetic, and a sympathetic component. The sensory root connects with the second division of the trigeminal nerve, and for the most part, the sensory branches of the SPG pass into the palatine nerves that innervate the mouth, soft palate, tonsils, and membranes lining the nasal cavity.9 It also provides sensory innervation to the retro-orbital and anterior inferior area of the calvarium. The parasympathetic root is distributed with trigeminal innervation to the nose and oral cavity structures and sends post ganglion fibers to the lacrimal nerve.
Nasal and pharyngeal glands are innervated by parasympathetic fibers of the SPG. The SPG also receives fibers from the superior cervical ganglion that form part of the carotid plexus (Fig. 1). Thus, from an anatomical and physiological perspective, there is a rationale to consider SPG blockade as an effective acute and preventative treatment for CM.
Fig 1.
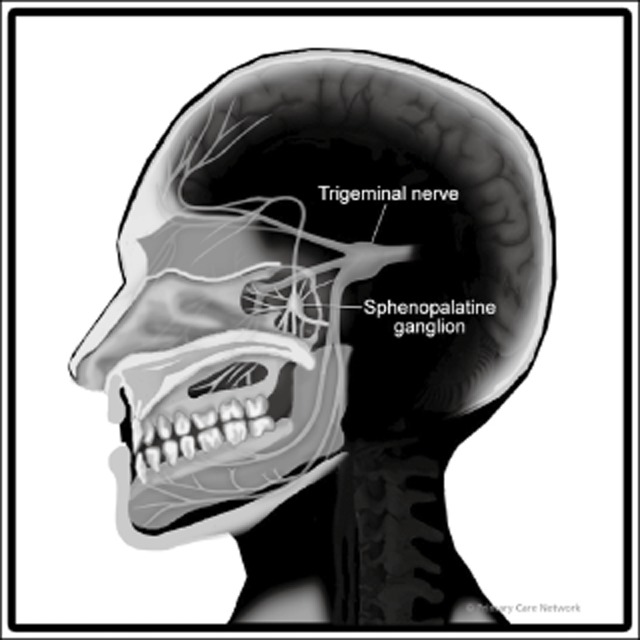
Sphenopalatine ganglion. Reproduced with permission from Primary Care Network©.
SPG Blockade
Several anatomic approaches are employed in an effort to anesthetize the SPG. Each has potential complications and technical challenges. The most common method for SPG blockade is the transnasal approach. With this approach, cotton pledgets or Q-tips soaked with an anesthetic agent are either passed through a nasal cannula or blindly with manual guidance to the nasopharynx. The challenge becomes getting the anesthetic agent to the PPF, as it lies lateral to the wall of the nasopharynx. Although SPG blockade via the transnasal approach is reported effective in some studies in migraine and cluster headache,10,11 the procedure does have potential adverse events (AEs) such as epistaxis, rare central nervous system (CNS) infections, and the certainty of getting an anesthetic agent to SPG is unpredictable.12
A second approach to the SPG is through the mouth or transoral approach. This is commonly employed by dentists. It is considered to be more technically challenging than the transnasal approach and again unpredictable in assuring anesthesia is delivered to the SPG. There is a risk of rare, but serious CNS infection associated with this procedure.
A third method is the infrazygomatic arch approach. In this procedure, generally conducted under fluoroscopic imaging, a cannula is guided percutaneously through the pterygomaxillary fissure in anatomic proximity to the PPF where an anesthetic agent is instilled through the cannula. This approach is considered a more accurate means of delivering anesthetic to the SPG, but also more technically challenging and requires considerable technical expertise. It is associated with rare, but potentially serious AEs such as meningitis and cardiac arrest.
In addition to the various approaches listed, more elaborate procedures are also performed including neurolytic SPG blockade with either surgery, alcohol injection, or radiofrequency ablation. While providing accurate localization to the SPG, neurolytic blockades are associated with significant complications and/or uncertainty in anesthetizing the SPG.13,14 Neurolytic approaches, while demonstrating effectiveness in small clinical studies, are also associated with significant potential complications and adverse consequences. Further, the procedure requires significant expertise/experience. Once successfully performed, nerve regeneration occurs within 3-6 months and thus it requires repeated procedures and exposure to the same risks.
Historically, there have been anecdotal reports of SPG blockade as a successful modality to treat migraine and cluster headache,15–18 although not all studies have demonstrated success.19 Recently, Tepper et al demonstrated efficacy of an implanted electrical stimulator in the SPG in a group of subjects with intractable CM.20 In the Tepper et al study of 11 subjects, 2 had complete resolution of headache within 3 minutes, 3 had a reduction in pain, 5 had no pain response, and 1 was not stimulated. The study supports potential roles of SPG in the treatment of intractable CM.
In this manuscript, we report on a repetitive procedure that provides SPG blockade as acute treatment for CM. A second manuscript assessing the impact of repetitive SPG blockade as a potentially disease-modifying treatment for CM and its longer-term impact on quality of life measures will be considered.
In this study, SPG blockade was accomplished with a novel device called the Tx360® by Tian Medical Inc. (Lombard, IL, USA). This device contains a small, flexible, soft plastic tube that is advanced below the middle turbinate just past the PPF. The plastic tube can then be rotated laterally on a preset track and extended into the intranasal space. A total of 0.3 cc of anesthetic (0.5% bupivacaine) is injected through the tube and directed to the mucosa covering the SPG.11 Dosing and anesthetic type was determined per device manufacturer's recommendations. The procedure is performed similarly in each nostril.
We hypothesized that repetitive SPG blocks with 0.5% bupivacaine delivered through the Tx360® device would be superior in reducing acute pain associated with CM compared with saline.
Methods
A double-blind, parallel-arm, placebo-controlled, randomized pilot study was conducted at 2 headache centers in the US. Due to lack of comparator studies available and the pilot design of this study, the sample size was estimated and a power analysis was not completed.
Protocol Approvals, Registrations, and Patient Consents
This study was conducted in accordance with the Declaration of Helsinki, all relevant US federal regulations, and in compliance with the International Conference on Harmonization guideline for Good Clinical Practice. The study protocol, informed consent forms, and any other appropriate study-related documents were reviewed and approved by Sterling Institutional Review Board/Ethics Committee. Written informed consent was obtained from each patient prior to any protocol-related activities. The study was a sponsor-initiated study funded by Tian Medical Inc., Lombard, IL, and reported on http://Clinicaltrials.gov (NCT01709708).
Design
Subjects were recruited through the use of flyers, web postings, radio ads, and current clinic patients at 2 headache specialty clinics located in Springfield, MO and Ann Arbor, MI. All subjects were screened using Institutional Review Board (IRB)-approved phone scripts. Subjects had a history of CM defined by the Headache Classification Committee of the International Headache Society ICHD-II appendix definition 2006, and by history, they had experienced CM for at least 3 months prior to enrollment. They were allowed to be on migraine preventive and abortive medications, provided that the dose was stable for 30 days prior to screening and agreed to not start, stop, or change medication and/or dosage during the study period. All headaches associated with the spectrum of CM were treated regardless of pain severity at the time of treatment.
Following a 28-day baseline period, subjects meeting inclusion and exclusion criteria were randomized at each study site by a supervisory individual who was not associated with the study subjects or visits. The randomization scheme was generated using the web site: (http://www.randomization.com). Forty-one subjects were randomized 2:1 to receive 0.5% bupivacaine or saline, respectively. The supervisory individual numbered and assigned study medication, based on the randomization plan, in a blinded fashion to subject, coordinator, and investigator.
Study Population
Fifty-five subjects were screened for study inclusion. Forty-nine met inclusion/exclusion criteria and were required to complete a daily headache diary for 28 days to confirm an accurate diagnosis of CM. Of the 49 subjects, 41 met diagnostic criteria for CM and were randomized 2:1 to receive a series of 12 SPG blocks with either 0.3 cc of 0.5% bupivacaine or saline delivered with the Tx360® over a 6-week period of time (2 SPG blocks/week). Pretreatment baseline headache pain scores for all randomized headache patients were determined at visit 2 prior to their first SPG block.
At each treatment visit (visits 2-13), vital signs and changes in medical, headache, and medication history were collected and subjects completed the numeric rating scale (NRS). At visit 2 and visit 14, subjects completed a Headache Impact Test (HIT-6) questionnaire.
At each treatment visit, subjects were administered an SPG block through each nostril, utilizing 0.5% bupivacaine or saline by the investigator or study coordinator using the Tx360® device. All subjects were given a piece of lemon candy as a taste distractor in an effort to maintain blinding prior to each procedure.
After each procedure, subjects completed the NRS at 15 and 30 minutes. At 30 minutes, subjects completed the Patient's Global Impression of Change (PGIC). Subjects also completed the NRS, PGIC, and a question about satisfaction at 24 hours. Daily diaries were completed throughout the active treatment period (Tables 1 and 2).
Table 1.
Study Timeline
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | Visit 9 | Visit 10 | Visit 11 | Visit 12 | Visit 13 | Visit 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Informed consent | X | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Physical exam | X | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Medical/migraine/medication history | X | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Randomization | — | X | — | — | — | — | — | — | — | — | — | — | — | — |
| Administer treatment | — | X | X | X | X | X | X | X | X | X | X | X | X | — |
| Update medications | — | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Dispense headache diary | X | X | — | — | — | — | — | — | — | — | — | — | X | — |
| Review headache diary | — | X | — | — | X | — | — | X | — | — | X | — | — | X |
| Administer HIT-6 | — | X | — | — | — | — | — | — | — | — | — | — | — | X |
| Administer before procedure, 15 minutes postquestionnaire, and 30 minutes postquestionnaire | — | X | X | X | X | X | X | X | X | X | X | X | X | — |
| Dispense 24-hour questionnaire | — | X | X | X | X | X | X | X | X | X | X | X | X | — |
| Collect adverse events | — | — | X | X | X | X | X | X | X | X | X | X | X | X |
| Complete source doc/CRF | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
—, not completed; X, completed; CRF, case report form; HIT-6, Headache Impact Test.
Table 2.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|---|
|
ICHD = International Classification of Headache Disorders, second edition.
Primary End-Point
To compare the NRS scores at pretreatment baseline vs 15 minutes, 30 minutes, and 24 hours postprocedure for all 12 treatments.
Secondary End-Points
To compare change in NRS score from preprocedure to 15 minutes, 30 minutes, and 24 hours postprocedure for all 12 treatments.
To compare 24 hours postprocedure PGIC score for all 12 treatments.
Acute medications usage over the active treatment phase.
AEs reported by all subjects during the active treatment phase.
HIT-6 scores pretreatment vs postfinal treatment.
Statistical Analysis
Data were collected electronically and analyzed using JMP, Version 8 (SAS Institute Inc., Cary, NC, USA), utilizing a repeated measures mixed-design analysis of variance (ANOVA) including a within-subjects factor of time (before treatment, 15 and 30 minutes posttreatment, and 24 hours posttreatment); and a between-subject factor of treatment groups (bupivacaine/sham saline) was also used to analyze data for statistical significance. Post-hoc analyses were conducted using 2 sample Wilcoxon rank sums. To control for multiple comparisons, Sidak corrections were calculated and a cut-off of P = .01 was used to determine statistical significance. Missing data were reviewed and found to be missing completely at random and less than 5% for any one subject or one variable. All data collected were included in data analysis if eligibility criteria were met, making this a per protocol analysis.
Results
Forty-one subjects randomized 2:1 for this study including 10 males and 31 females with a mean age of 41.3 years and a diagnosis of CM (Table 3, Fig. 2). Of the study population, 83% were Caucasian, 10% African American, and 7% Other. During the treatment period, one subject withdrew consent in the saline group due to lack of efficacy. A total of 40 subjects completed treatment in the bupivacaine and sham saline groups: 27 and 13, respectively. Three subjects, one in the bupivacaine group and 2 in the sham saline group, were removed from data analysis due to protocol violations. The final data set included 38 subjects, 26 in the bupivacaine group (including the one subject that did not complete treatment) and 12 in the sham saline group. Subjects were diagnosed with CM on average 8.58 years prior to the start of the study and had an average of 23.63 headaches per month, with 15.24 of them being classified with a migraine phenotype (Table 4).
Table 3.
Subject Demographics
| Total (N = 41) | Bupivacaine (n = 27) | Saline (n = 14) | |
|---|---|---|---|
| Gender | |||
| Male | n = 10 (24.4%) | n = 7 (25.9%) | n = 3 (21.4%) |
| Female | n = 31 (75.6%) | n = 20 (74.1%) | n = 11 (78.6%) |
| Age (years) | |||
| Mean | 41.30 | 40.96 | 41.97 |
| Standard deviation | 12.59 | 11.63 | 14.71 |
| Range (min, max) | 18, 67 | 22, 63 | 18, 67 |
| Ethnicity | |||
| Caucasian | n = 34 (82.9%) | n = 20 (74.1%) | n = 14 (100%) |
| African American | n = 4 (9.8%) | n = 4 (14.8%) | n = 0 (0%) |
| Other | n = 3 (7.3%) | n = 3 (11.1%) | n = 0 (0%) |
Fig 2.
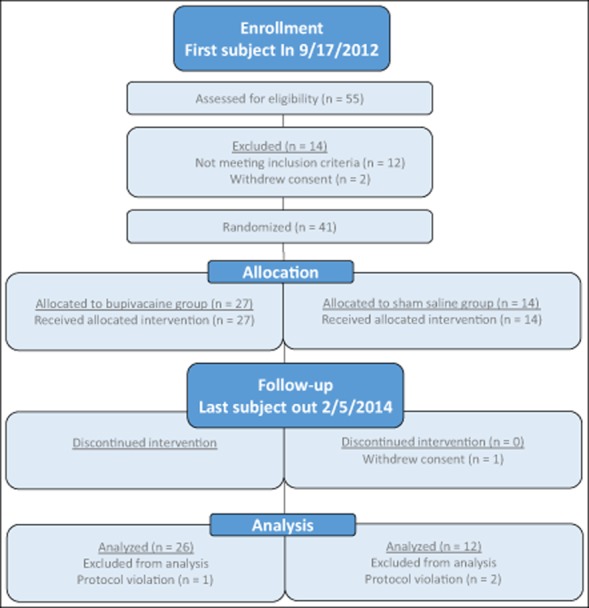
Study flow diagram.
Table 4.
Migraine Characteristics
| Total (M) | Bupivacaine (M) | Saline (M) | |
|---|---|---|---|
| Chronic migraine diagnosis duration (years) | 8.58 | 8.78 | 8.20 |
| Baseline headache characteristics | |||
| Migraine days | 15.2 | 15 | 15.8 |
| Headache days | 23.6 | 23.1 | 24.8 |
The primary end-point of the study was to compare NRS scores at baseline, and 15 minutes, 30 minutes, and 24 hours posttreatment between SPG blockades with 0.5% bupivacaine vs sham saline. When pooling all of the treatments 1-12, a repeated measures ANOVA showed a statistically significant interaction of time and group NRS scores over time, F(3, 438) = 4.90, P = .002. Results also revealed a statistically significant main effect of time (F[3, 438] = 29.34, P < .001) and group (F[1, 440] = 18.61, P < .001) (Fig. 3). Subjects receiving treatment with bupivacaine experienced a statistically significant reduction in the NRS scores compared with those receiving saline at baseline, 15 minutes, 30 minutes, and 24 hours after treatment (Fig. 3, Table 5). Subjects treated with bupivacaine experienced a statistically significant decrease from their baseline scores 15 minutes (Mdiff = −0.65, P < .001) and 30 minutes (Mdiff = −0.77, P < .001) after treatment and they continued to experience relief 24 hours after the procedure (Mdiff = −0.29, P = .02). Although subjects receiving sham saline did experience a statistically significant decrease from baseline in NRS scores at 15 minutes (Mdiff = −0.27, P < .001) and 30 minutes (Mdiff = −0.33, P < .001), by 24 hours a statistically significant increase from baseline was found (Mdiff = 0.42, P = .045).
Fig 3.
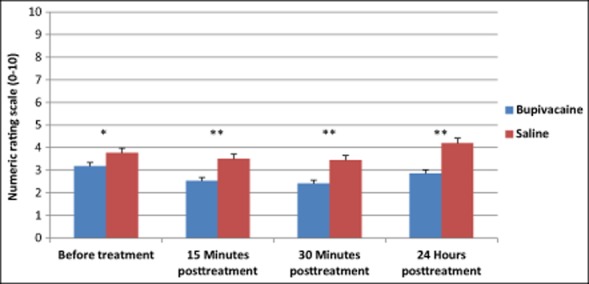
Treatment with bupivacaine delivered with the Tx360® device reduced average pooled numeric rating scale (NRS) scores compared with those treated with saline. Average NRS scores from all treatments pooled are shown. Between group P values: Before treatment *P = .010; 15 minutes, 30 minutes, and 24 hours posttreatment **P < .001.
Table 5.
Numeric Rating Scale Scores for Pooled Treatments 1-12
| n | M (SD) | P value | ||
|---|---|---|---|---|
| Before treatment | Bupivacaine | 26 | 3.18 (2.79) | .010* |
| Saline | 12 | 3.78 (2.48) | ||
| 15 Minutes posttreatment | Bupivacaine | 26 | 2.53 (2.61) | <.001** |
| Saline | 12 | 3.51 (2.39) | ||
| 30 Minutes posttreatment | Bupivacaine | 26 | 2.41 (2.61) | <.001** |
| Saline | 12 | 3.45 (2.36) | ||
| 24 Hours posttreatment | Bupivacaine | 26 | 2.85 (2.74) | <.001** |
| Saline | 12 | 4.20 (2.62) |
P ≤ .05;
P ≤ .001.
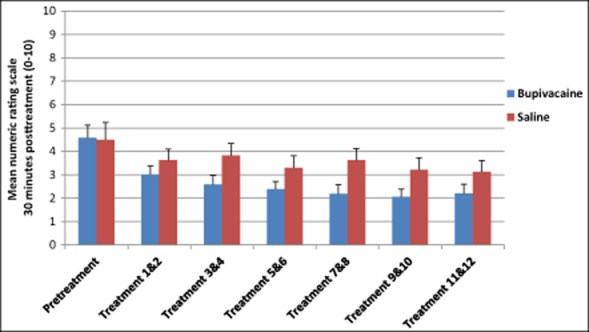
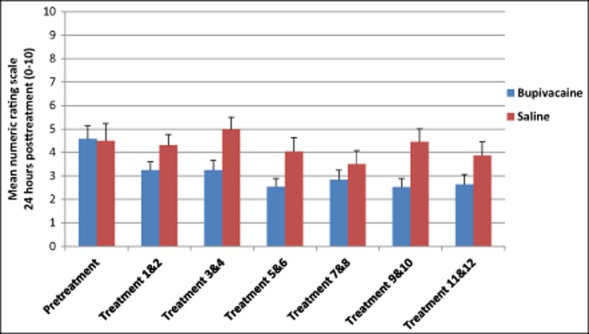
To establish the effect of repeated sphenopalatine blocks and to confirm differences in the NRS pooled were not solely a regression to the mean, further analysis examining changes in the NRS through each of the treatment cycles was conducted. Although not statistically significant, there was a decreasing trend in the bupivacaine group across the 12 treatments compared with those receiving sham saline 15 minutes posttreatment (Fig. 4, Table 6). A similar trend was seen in NRS scores 30 minutes and 24 hours after treatment (Figs. 5 and 6 and Tables 7 and 8).
Fig 4.
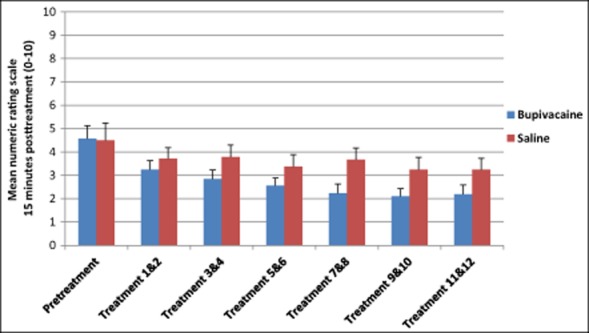
Average numeric rating scale scores 15 minutes after repetitive sphenopalatine blocks with either bupivacaine or sham saline.
Table 6.
Numeric Rating Scale Scores 15 Minutes Posttreatment
| n | M (SD) | ||
|---|---|---|---|
| Pretreatment | Bupivacaine | 26 | 4.78 (2.76) |
| Saline | 12 | 4.50 (2.43) | |
| Treatment 1 & 2 | Bupivacaine | 26 | 3.25 (2.59) |
| Saline | 12 | 3.71 (2.18) | |
| Treatment 3 & 4 | Bupivacaine | 26 | 2.85 (2.75) |
| Saline | 12 | 3.79 (2.45) | |
| Treatment 5 & 6 | Bupivacaine | 26 | 2.56 (2.37) |
| Saline | 12 | 3.38 (2.58) | |
| Treatment 7 & 8 | Bupivacaine | 26 | 2.23 (2.65) |
| Saline | 12 | 3.67 (2.41) | |
| Treatment 9 & 10 | Bupivacaine | 26 | 2.10 (2.36) |
| Saline | 12 | 3.25 (2.45) | |
| Treatment 11 & 12 | Bupivacaine | 25 | 2.18 (2.82) |
| Saline | 12 | 3.25 (2.44) |
SD = standard deviation.
Fig 5.
Average numeric rating scale scores 30 minutes after repetitive sphenopalatine blocks with either bupivacaine or saline.
Fig 6.
Average numeric rating scale scores 24 hours after repetitive sphenopalatine blocks with either bupivacaine or saline.
Table 7.
Numeric Rating Scale Scores 30 Minutes Posttreatment
| n | M (SD) | ||
|---|---|---|---|
| Pretreatment | Bupivacaine | 26 | 4.58 (2.76) |
| Saline | 12 | 4.50 (2.43) | |
| Treatment 1 & 2 | Bupivacaine | 26 | 3.12 (2.65) |
| Saline | 12 | 3.63 (2.28) | |
| Treatment 3 & 4 | Bupivacaine | 26 | 2.60 (2.70) |
| Saline | 12 | 3.83 (2.43) | |
| Treatment 5 & 6 | Bupivacaine | 26 | 2.39 (2.31) |
| Saline | 12 | 3.29 (2.46) | |
| Treatment 7 & 8 | Bupivacaine | 26 | 2.19 (2.77) |
| Saline | 12 | 3.63 (2.39) | |
| Treatment 9 & 10 | Bupivacaine | 26 | 2.06 (2.38) |
| Saline | 12 | 3.21 (2.48) | |
| Treatment 11 & 12 | Bupivacaine | 25 | 2.20 (2.81) |
| Saline | 12 | 3.13 (2.31) |
SD = standard deviation.
Table 8.
Numeric Rating Scale Scores 24 Hours Posttreatment
| n | M (SD) | ||
|---|---|---|---|
| Pretreatment | Bupivacaine | 26 | 4.58 (2.76) |
| Saline | 12 | 4.50 (2.43) | |
| Treatment 1 & 2 | Bupivacaine | 26 | 3.24 (2.53) |
| Saline | 12 | 4.30 (2.14) | |
| Treatment 3 & 4 | Bupivacaine | 26 | 3.25 (2.88) |
| Saline | 12 | 5.00 (2.41) | |
| Treatment 5 & 6 | Bupivacaine | 26 | 2.54 (2.53) |
| Saline | 12 | 4.04 (2.84) | |
| Treatment 7 & 8 | Bupivacaine | 26 | 2.84 (2.93) |
| Saline | 12 | 3.50 (2.81) | |
| Treatment 9 & 10 | Bupivacaine | 26 | 2.52 (2.55) |
| Saline | 12 | 4.46 (2.65) | |
| Treatment 11 & 12 | Bupivacaine | 25 | 2.63 (2.95) |
| Saline | 12 | 3.88 (2.77) |
NRS scores from each subject were normalized to the pretreatment values, and the percent changes from pretreatment values of all treatments pooled were determined. Results from a repeated measures ANOVA showed a statistically significant main effect of time (F[2,349] = 9.53, P < .001) and group (F[1,350] = 34.54, P < .001) for percent change of NRS scores. Subjects in the bupivacaine group reported a statistically significant greater decrease in pain severity at 15 minutes, 30 minutes, and 24 hours after treatment than those receiving only saline (Fig. 7, Table 9).
Fig 7.
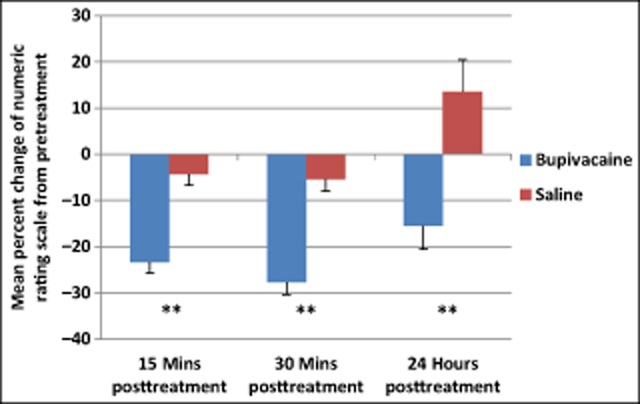
Subjects treated with bupivacaine had a statistically significant greater decrease in pooled numeric rating scale scores from baseline compared to those receiving sham saline. Between group P values: 15 minutes, 30 minutes, 24 hours posttreatment P < .001.
Table 9.
Numeric Rating Scale Percent Change
| M (SD) | P value | ||
|---|---|---|---|
| 15 Minutes posttreatment | Bupivacaine | −23.3 (34.5) | <.001** |
| Saline | −4.31 (27.3) | ||
| 30 Minutes posttreatment | Bupivacaine | −27.7 (37.1) | <.001** |
| Saline | −5.41 (29.2) | ||
| 24 Hours posttreatment | Bupivacaine | −15.5 (69.9) | <.001** |
| Saline | 13.5 (78.8) |
*P ≤ .05;
P ≤ .001.
Results from a repeated measures ANOVA revealed a statistically significant between-subject difference in PGIC scores over time, F(1,432) = 66.86, P < .001. Subjects receiving bupivacaine reported statistically significant lower PGIC scores than those receiving saline, indicating greater impression of improvement at both 30 minutes (M = mean, SD = standard deviation; M = 3.00, SD = 1.02 vs M = 3.72, SD = 0.53, P < .001) and 24 hours (M = 3.08, SD = 1.26, vs M = 3.88, SD = 1.02, P < .001) posttreatment, respectively. Within-subject comparisons revealed a statistically significant increase in PGIC scores for the saline group, P = .04, indicating a worsening of the condition.
Average acute medication usage during the 4-week baseline period for saline and bupivacaine was similar (M = 23.3, SD = 22.1, vs M = 17.6, SD = 12.5; Z = 0.35, P = .73). During the 6-week treatment period, average acute medication usage was also similar between saline and bupivacaine (M = 32.1, SD = 44.6, vs M = 18.2, SD = 16.9; Z = 0.25, P = .80).
The procedure was well tolerated in both groups, and there was no statistically significant difference in AE reporting between the bupivacaine and saline group, with an average of 7.52 (SD = 8.16) events in the bupivacaine group and 5 (SD = 7.06) events in the saline-treated group (Z = −1.19, P = .23). The most common AEs for those treated with bupivacaine were mouth numbness (18%), lacrimation (29%), and bad taste (15%) (Table 10). There were no statistically significant differences found in the frequency of reporting of these 3 AEs between bupivacaine and saline groups (X2 = 3.56, P = .06; X2 = 2.65, P = .10; X2 = 1.44, P = .23), respectively. To further investigate the influence of AEs on the primary end-point, analysis was completed by comparing any subject who experienced mouth numbness, lacrimation, or bad taste to those who did not. No statistically significant differences were found between these 2 groups, F(33, 1254) = 0.71, P = .89.
Table 10.
Adverse Events
| Total |
Bupivacaine |
Saline |
||||
|---|---|---|---|---|---|---|
| Subject Frequency | Event Frequency | Subject Frequency | Event Frequency | Subject Frequency | Event Frequency | |
| f (%) | f (%) | f (%) | f (%) | f (%) | f (%) | |
| Anxiety | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Bad taste | 8 (20) | 33 (12) | 7 (26) | 30 (15) | 1 (7) | 3 (4) |
| Blurred vision | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Body pain | 2 (5) | 2 (1) | 2 (7) | 2 (1) | 0 (0) | 0 (0) |
| Cold | 9 (22) | 9 (3) | 7 (26) | 7 (3) | 2 (14) | 2 (3) |
| Congestion | 2 (5) | 2 (1) | 2 (7) | 2 (1) | 0 (0) | 0 (0) |
| Cough | 1 (2) | 2 (1) | 1 (4) | 2 (1) | 0 (0) | 0 (0) |
| Diarrhea | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Dizziness | 2 (5) | 5 (2) | 0 (0) | 0 (0) | 2 (14) | 5 (7) |
| Drowsiness | 1 (2) | 10 (4) | 0 (0) | 0 (0) | 1 (7) | 10 (14) |
| Ear infection | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Ear pain | 1 (2) | 10 (4) | 0 (0) | 0 (0) | 1 (7) | 10 (14) |
| Ear ringing | 2 (5) | 2 (1) | 0 (0) | 0 (0) | 2 (14) | 2 (3) |
| Elevated blood pressure | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Eye infection | 2 (5) | 2 (1) | 2 (7) | 2 (1) | 0 (0) | 0 (0) |
| Eye sting | 1 (2) | 12 (4) | 0 (0) | 0 (0) | 1 (7) | 12 (17) |
| Facial numbness | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Facial pain | 2 (5) | 4 (1) | 2 (7) | 4 (2) | 0 (0) | 0 (0) |
| General numbness | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Head pain | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Heartburn | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Indigestion | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Insomnia | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Lacrimation | 9 (22) | 59 (22) | 8 (30) | 58 (29) | 1 (7) | 1 (1) |
| Laryngitis | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Light headed | 3 (7) | 4 (1) | 1 (4) | 1 (<1) | 2 (14) | 3 (4) |
| Low back pain | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Mouth numbness | 6 (15) | 37 (14) | 6 (22) | 37 (18) | 0 (0) | 0 (0) |
| Nasal bleeding | 3 (7) | 3 (1) | 2 (7) | 2 (1) | 1 (7) | 1 (1) |
| Nasal drainage | 2 (5) | 23 (8) | 2 (7) | 23 (11) | 0 (0) | 0 (0) |
| Nasal irritation | 7 (17) | 13 (5) | 4 (15) | 7 (3) | 3 (21) | 6 (9) |
| Nasal swelling | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Nausea | 1 (2) | 8 (3) | 1 (4) | 8 (4) | 0 (0) | 0 (0) |
| Neck pain | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Numb throat | 1 (2) | 2 (1) | 1 (4) | 2 (1) | 0 (0) | 0 (0) |
| Occipital pain | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Palpitations | 1 (2) | 2 (1) | 1 (4) | 1 (<1) | 1 (7) | 1 (1) |
| Pneumonia | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Sinus infection | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Sinus inflammation | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Sinus numbness | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Sneeze | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Sore throat | 4 (10) | 4 (1) | 2 (7) | 2 (1) | 2 (14) | 2 (3) |
| Sprained ankle | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Thumb laceration | 1 (2) | 1 (<1) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Tingling | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
| Tooth abscess | 1 (2) | 1 (<1) | 1 (4) | 1 (<1) | 0 (0) | 0 (0) |
A serious AE did occur during the posttreatment follow-up phase of the study. One subject, who received saline, experienced a pulmonary embolism, which resulted in death 81 days postfinal treatment. This SAE was determined not be related to the study treatment.
HIT-6 scores did not indicate a between-group interaction from before treatment to the final treatment. However, HIT-6 scores were statistically decreased in subjects receiving treatments with bupivacaine from before treatment to the final treatment (Mdiff = −4.52 [95% confidence interval {CI}: 0.29 to −3.38], P = .005), whereas no statistically significant change was seen in the sham saline group (Mdiff = −1.50 [95% CI: −1.56 to −7.94], P = .13) (Fig. 8).
Fig 8.
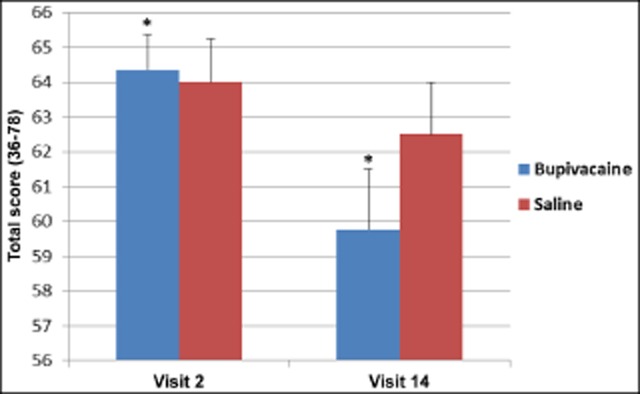
Repetitive sphenopalatine blocks with bupivacaine caused a statistically significant decrease in Headache Impact Test-6 scores, P = .005*; bupivacaine n = 25, saline n = 11.
Discussion
This pilot study illustrates that SPG blockade with 0.3 cc of 0.5% bupivacaine delivered by the Tx360® device has superior efficacy to sham saline blockade as a repetitive acute treatment for headache pain associated with CM. The benefit was observed at both the 15 and 30 minute postprocedure time points and sustained at the 24-hour time point. Over the 6-week injection cycle, there was a statistically significant reduction of pretreatment NRS scores with successive SPG block using bupivacaine, but not sham saline. There was also sustained improvement in PGIC at 24 hours posttreatment and statistically significant improvement in HIT-6 scores comparing pretreatment scores vs postfinal treatment scores.
The SPG blockade procedure with the Tx360® was well tolerated whether delivering bupivacaine or saline. There were few systemic AEs for either group. The most common AEs were lacrimation, unpleasant taste, and mouth numbness, although the increase in frequency in reporting in the treatment group did not significantly affect the primary outcome. All AEs were considered mild to moderate, short in duration, and resolved spontaneously. No subject withdrew because of AEs.
There is a clinical need to better understand the efficacy and clinical utility of acute and preventive treatment for patients with CM. This is challenging given the paradigms for clinical study design of acute treatment of migraine were largely developed for study of EM. Although generally, pharmacological treatment of migraine is divided into acute and prophylactic, the distinction between acute and preventative treatments becomes less clear as migraine frequency increases. For example, in EM, the optimal definition of success for acute treatment (supported by the IHS) is 2-hour pain freedom or 24-hour sustained pain freedom.21 This 2-hour pain free paradigm works well to measure a decrease in attack duration when a headache is destined to terminate within a defined time span. However, in CM, the benefit of a pain-free outcome is more elusive as patients may not experience headache termination and often return to a prolonged low-grade baseline headache, following acute treatment. Even when patients become pain-free, it is often for a brief time period and followed in close proximity by the emergence of more headache events. When sustained, a reduction of headache often represents welcomed clinically meaningful relief.
This clinical dilemma is illustrated in this study as the study population was experiencing nearly 25 days of headache per 28-day period through baseline, suggesting that pain-free days, while undoubtedly desirable, were uncommon. SPG blockade with bupivacaine produced sustained relief to 24 hours postprocedure and with repeated SPG blockade was associated with continued reduction of the pretreatment baseline headache pain over the study period. Sustained benefit of this statistically significant relief was reflected in the reduction in HIT-6 scores for SPG blockade with bupivacaine, but not saline. It may be that an appropriate end-point for acute treatment of CM is better defined by patient-centered standards based on what is meaningful to the patient and analysis of improvement in the overall pattern of headache rather than a single time point following a treatment intervention.
Additionally, there is a clear clinical need to have alternative treatments for acute intervention that do not put CM patients at risk for MOH or at the very least can be used in conjunction with other acute drug interventions to reduce exposure to medications associated with MOH. Both clinical trials and epidemiological studies suggest that most CM patients use acute medication at frequencies above those defined as MO by ICHD-III beta.22,23 Although repeated exposure to bupivacaine has to date not been associated with MOH, further studies will be required before this can be stated conclusively. However, we hypothesize that the use of SPG blockade may represent a viable alternative to increasing the use of acute medication. This may be a particularly good option given the overall benefit observed with the completion of the series of SPG blocks, high degree of tolerability, and ease of administration. Clearly, SPG blockade in CM patients warrants further investigation.
There are numerous limitations to this study. First, the AEs of lacrimation, taste, and oral numbing in subjects receiving bupivacaine may suggest that study blinding was not maintained for all subjects. A lemon candy was used as a taste distractor for the subjects, but it did not distract them from taste discrimination in all cases and could not prevent mouth numbness or lacrimation. We conducted an analysis of first treatment results in an effort to minimize unblinding of the study by the experiences of AEs. In the first treatment analysis, the efficacy of SPG with active medication was similar to that observed in the pooled data. Furthermore, the primary end-point was not statistically different when comparing those who experience AEs to those who did not. Other limitations included a small sample size of the study population and an inability to control for headache intensity at the time of treatment due to difficulties in the scheduling of CM subjects for multiple treatments in a specific time frame. Controlling for these variables would be of interest in future studies.
In addition, no effort was made to identify a subpopulation of subjects with CM that may have been more appropriate for SPG blockade. In retrospect, we might have delineated further between MO and MOH patients, and this too is a limitation to the study. For example, subjects with frontal headaches or subjects with or without MO may have responded differently to repetitive SPG blockade than the general CM population. Further limitations of the study include the exploratory nature of its design. The optimal frequency and duration of SPG blockade in CM is unknown. Additionally, there was neither control of comorbidities nor usage of abortive medications before treatments that might affect efficacy. Finally, there are no studies of the long-term safety of repetitive SPG blockade. These limitations provide opportunities for further study and research.
Conclusion
Recognizing the limitations of this study, we conclude that these data suggest that SPG blockade with bupivacaine delivered repetitively with the Tx360® device appears to demonstrate early potential as an acute treatment of headache in some subjects with CM. Statistically significant headache relief is noted at 15 and 30 minutes and sustained at 24 hours for SPG blockade with bupivacaine vs saline. The Tx360® device was simple to use and not associated with any statistically significant or lasting AEs. Further research on the efficacy, optimal frequency, and numbers of repetitive SPG blockade is warranted.
Glossary
- AE
adverse event
- ANOVA
analysis of variance
- CI
confidence interval
- CM
chronic migraine
- CNS
central nervous system
- EM
episodic migraine
- HIT-6
Headache Impact Test
- ICHD-II
International Classification of Headache Disorders, second edition
- ICHD-III
The International Classification of Headache Disorders, third edition (beta version)
- IHS
International Headache Society;
- MO
medication overuse
- MOH
medication overuse headache
- NRS
numeric rating scale
- PGIC
Patient's Global Impression of Change
- PPF
pterygopalatine fossa
- SD
standard deviation
- SPG
sphenopalatine ganglion
Statement of Authorship
Category 1
-
(a) Conception and Design
Roger Cady; Joel Saper
-
(b) Acquisition of Data
Roger Cady; Joel Saper; Kent Dexter
-
(c) Analysis and Interpretation of Data
Roger Cady; Heather R. Manley; Joel Saper
Category 2
-
(a) Drafting the Manuscript
Roger Cady; Joel Saper; Heather R. Manley
-
(b) Revising It for Intellectual Content
Roger Cady; Joel Saper; Heather R. Manley; Kent Dexter
Category 3
-
(a) Final Approval of the Completed Manuscript
Roger Cady; Joel Saper; Heather R. Manley; Kent Dexter
References
- 1.Stovner LJ, Hagen K, Jensen R. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 2.Cady RK, Schreiber CP, Farmer KU. Understanding the patient with migraine: The evolution from episodic headache to chronic neurological disease. Headache. 2004;44:426–435. doi: 10.1111/j.1526-4610.2004.04094.x. [DOI] [PubMed] [Google Scholar]
- 3.Katsarava Z, Schneeweiss S, Kurth T. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62:788–790. doi: 10.1212/01.wnl.0000113747.18760.d2. [DOI] [PubMed] [Google Scholar]
- 4.Silberstein SD, Lipton RB, Saper JR. Chronic daily headache including transformed migraine, chronic tension-type headache, and medication overuse headache. In: Silberstein SD, Lipton RB, Dodick DW, editors. Wolff's Headache and Other Head Pain. 8th edn. New York, NY: Oxford University Press; 2008. pp. 315–377. [Google Scholar]
- 5.Headache Classification Committee. Olesen J, Bousser MG. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 6.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 7.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia. 2004;24(Suppl. 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Hazard E, Muakata J, Bigal ME, Rupnow MFT, Lipton RB. The burden of migraine in the United States: Current and emerging perspectives on disease management and economic analysis. Value Health. 2009;12:55–64. doi: 10.1111/j.1524-4733.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Syed MI, Shaikh A. Radiology of Non-Spinal Pain Procedures: A Guide for the Interventionalist. Berlin, Germany: Springer; 2011. pp. 5–6. [Google Scholar]
- 10.Maizels M, Geiger AM. Intranasal lidocaine for migraine: A randomized trial and open-label followup. Headache. 1999;39:543–551. doi: 10.1046/j.1526-4610.1999.3908543.x. [DOI] [PubMed] [Google Scholar]
- 11.Candido KD, Massey ST, Sauer R, Darabad RR, Knezevic NN. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician. 2013;16:E769–E778. [PubMed] [Google Scholar]
- 12.Yang I, Oraee S. A novel approach to transnasal sphenopalatine ganglion injection. Pain Physician. 2006;9:131–134. [PubMed] [Google Scholar]
- 13.Piagkou M, Demesticha T, Troupis T. The pterygopalatine ganglion and its role in various pain syndromes: From anatomy to clinical practice. Pain Pract. 2012;12:399–412. doi: 10.1111/j.1533-2500.2011.00507.x. [DOI] [PubMed] [Google Scholar]
- 14.Narouze S, Kapural L, Casanova J, Mekhail N. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache. 2009;49:571–577. doi: 10.1111/j.1526-4610.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 15.Paemeleire K, Goodman AM. Results of a patient survey for an implantable neurostimulator to treat migraine headaches. J Headache Pain. 2012;13:239–241. doi: 10.1007/s10194-012-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansarinia M, Rezai A, Tepper SJ. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache. 2010;50:1164–1174. doi: 10.1111/j.1526-4610.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- 17.Schoenen J, Jensen RH, Lantéri-Minet M. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia. 2013;33:816–830. doi: 10.1177/0333102412473667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudrow L, Kudrow DB, Sandweiss JH. Rapid and sustained relief of migraine attacks with intranasal lidocaine: Preliminary findings. Headache. 1995;35:79–82. doi: 10.1111/j.1526-4610.1995.hed3502079.x. [DOI] [PubMed] [Google Scholar]
- 19.Blanda M, Rench T, Gerson LW, Weigand JV. Intranasal lidocaine for the treatment of migraine headache: A randomized, controlled trial. Acad Emerg Med. 2001;8:337–342. doi: 10.1111/j.1553-2712.2001.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 20.Tepper SJ, Rezai A, Narouze S, Steiner C, Mohajer P, Ansarinia M. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache. 2009;49:983–989. doi: 10.1111/j.1526-4610.2009.01451.x. [DOI] [PubMed] [Google Scholar]
- 21.Tfelt-Hansen P, Pascual J, Ramadan N International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia. 2012;32:6–38. doi: 10.1177/0333102411417901. [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB, Bigal ME. Ten lessons on the epidemiology of migraine. Headache. 2007;47(Suppl. 1):S2–S9. doi: 10.1111/j.1526-4610.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 23.Cady R, O'Carroll P, Dexter K, Freitag F, Shade C. SumaRT/Nap vs naproxen sodium in treatment and disease modification of migraine: A pilot study. Headache. 2013;54:67–79. doi: 10.1111/head.12211. [DOI] [PubMed] [Google Scholar]


