Abstract
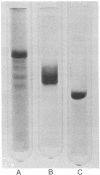
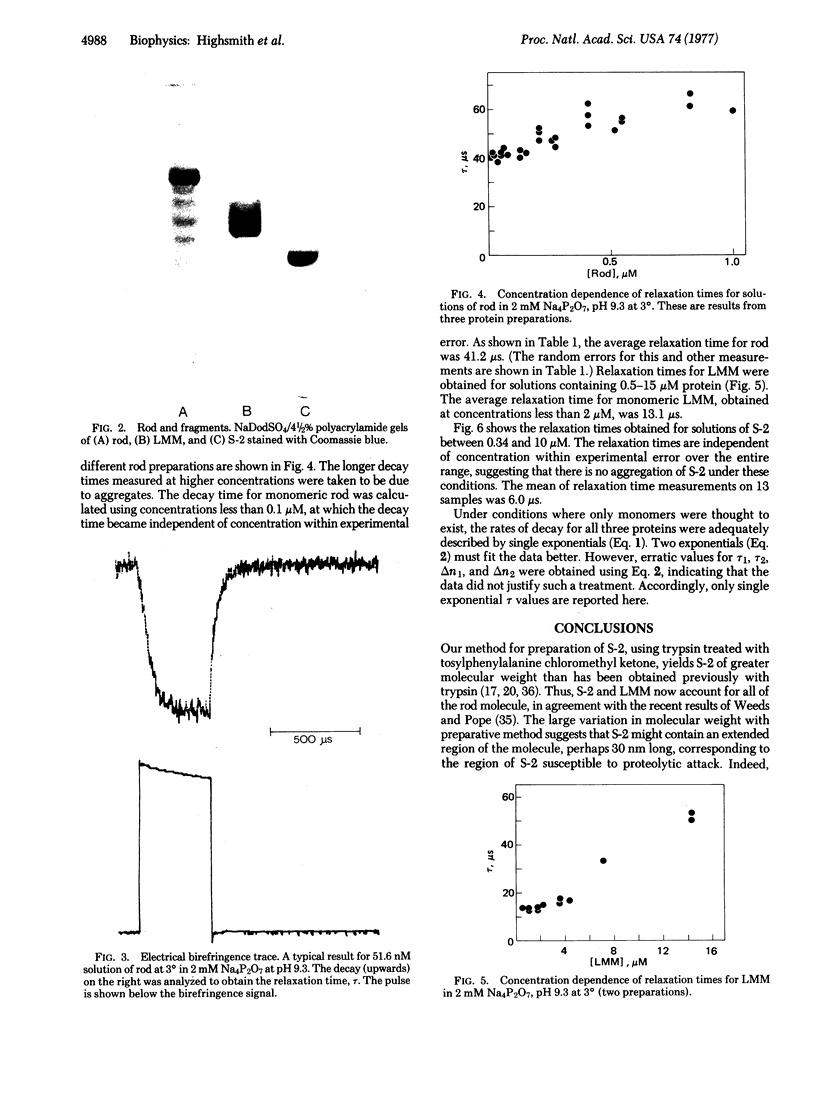
Myosin rod was prepared by papain proteolysis of myosin. The components of rod, light meromyosin (LMM) and subfragment-2 (S-2), were prepared by proteolysis of myosin and rod, respectively, using trypsin treated with tosylphenylalanine chloromethyl ketone. S-2, thus prepared, was of greater molecular weight than obtained previously, so that the combined molecular weights of LMM and S-2 were equal to that of rod, and S-2 contained virtually all of the region of the rod susceptible to trypsin. Electro-optical measurements were made on the three fragments in 2 mM sodium pyrophosphate, pH 9.3 at 3 degrees, over a large range of protein concentrations. Analysis of the relaxation of birefringence, at low protein concentration where there was no aggregation, showed that LMM (relaxation time 13.1 micros) behaves as a rigid cylinder. Rod (relaxation time 41.2 micros) and S-2 (relaxation time 6.0 micros) had relaxation rates that were too fast for rigid molecules of their dimensions, and therefore are not straight rods. This implies that myosin rod is flexible in the S-2 portion, presumably in the region susceptible to proteolysis. The implications of rod flexibility for the mechanism of muscle contraction are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAHMS J., BREZNER J. Interaction of myosin A with ions. Arch Biochem Biophys. 1961 Nov;95:219–228. doi: 10.1016/0003-9861(61)90138-2. [DOI] [PubMed] [Google Scholar]
- Burke M., Himmelfarb S., Harrington W. F. Studies on the "hinge" region of myosin. Biochemistry. 1973 Feb;12(4):701–710. doi: 10.1021/bi00728a020. [DOI] [PubMed] [Google Scholar]
- Bálint M., Menczel L., Fejes E., Szilágyi L. Studies on proteins and protein complexes of muscle by means of proteolysis. IX. Digestion of myosin by dissolved papain. Acta Biochim Biophys Acad Sci Hung. 1972;7(3):215–226. [PubMed] [Google Scholar]
- Bálint M., Sréter F. A., Gergely J. Fragmentation of myosin by papain--studies on myosin from adult fast and slow skeletal and cardiac, and embryonic muscle. Arch Biochem Biophys. 1975 Jun;168(2):557–566. doi: 10.1016/0003-9861(75)90287-8. [DOI] [PubMed] [Google Scholar]
- Cassim J. Y., Lin T. I. Does myosin-substrate interaction in vitro result in a delocalized conformation change? J Supramol Struct. 1975;3(5-6):510–519. doi: 10.1002/jss.400030510. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Goodno C. C., Harris T. A., Swenson C. A. Thermal transitions of myosin and its helical fragments. Regions of structural instability in the myosin molecule. Biochemistry. 1976 Nov 16;15(23):5157–5160. doi: 10.1021/bi00668a032. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRINGTON W. F., VON HIPPEL P. H., MIHALYI E. Proteolytic enzymes as probes of the secondary structure of fibrous proteins. Biochim Biophys Acta. 1959 Mar;32(1):303–304. doi: 10.1016/0006-3002(59)90601-8. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Himmelfarb S. Effect of adenosine di- and triphosphates on the stability of synthetic myosin filaments. Biochemistry. 1972 Aug 1;11(16):2945–2952. doi: 10.1021/bi00766a004. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- King M. V. Electron-microscopic mapping of the hinge region of myosin. Experientia. 1976 Aug 15;32(8):975–976. doi: 10.1007/BF01933919. [DOI] [PubMed] [Google Scholar]
- Kobayasi S., Totsuka T. Electric birefringence of myosin subfragments. Biochim Biophys Acta. 1975 Feb 17;376(2):375–385. doi: 10.1016/0005-2728(75)90029-8. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. Studies on the tryptic digestion of heavy meromyosin. Biochim Biophys Acta. 1961 Jul 8;50:599–601. doi: 10.1016/0006-3002(61)90030-0. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- Nihel T., Mendelson R. A., Botts J. The site of force generation in muscle contraction as deduced from fluorescence polarization studies. Proc Natl Acad Sci U S A. 1974 Feb;71(2):274–277. doi: 10.1073/pnas.71.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'KONSKI C. T., ZIMM B. H. New method for studying electrical orientation and relaxation effects in aqueous colloids; preliminary results with tobacco mosaic virus. Science. 1950 Feb;111(2875):113–116. doi: 10.1126/science.111.2875.113. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. The structure of synthetic polypeptides. Proc Natl Acad Sci U S A. 1951 May;37(5):241–250. doi: 10.1073/pnas.37.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe F. A. The myosin filament. I. Structural organization from antibody staining observed in electron microscopy. J Mol Biol. 1967 Jul 28;27(2):203–225. doi: 10.1016/0022-2836(67)90016-2. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Stone D. B. Interaction of spin-labeled myosin with substrate. Arch Biochem Biophys. 1970 Nov;141(1):378–380. doi: 10.1016/0003-9861(70)90148-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. L. Birefringence changes in vertebrate striated muscle. J Supramol Struct. 1975;3(2):181–191. doi: 10.1002/jss.400030212. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]