Abstract
Sphingolipids are ubiquitous building blocks of eukaryotic cell membranes. Progress in our understanding of sphingolipid metabolism, state-of-the-art sphingolipidomic approaches and animal models have generated a large body of evidence demonstrating that sphingolipid metabolites, particularly ceramide and sphingosine-1-phosphate, are signalling molecules that regulate a diverse range of cellular processes that are important in immunity, inflammation and inflammatory disorders. Recent insights into the molecular mechanisms of action of sphingolipid metabolites and new perspectives on their roles in regulating chronic inflammation have been reported. The knowledge gained in this emerging field will aid in the development of new therapeutic options for inflammatory disorders.
In the 1880s, the neurochemist J. L. W. Thudichum presciently named the brain lipid ‘sphingosine’ after the Sphinx, owing to its enigmatic chemical nature1. Sphingosine and its relatives continue to surprise and confound us today. These fatty amino alcohols are the backbone of a ubiquitous class of eukaryotic lipids, the sphingolipids, which include ceramide (N-acyl-sphingosine), sphingomyelin, and hundreds of different glycosphingolipids (Fig. 1a). Sphingolipids, like glycerolipids, are reservoirs of bioactive metabolites of profound importance to myriad cell signalling and pathological functions. The sphingolipid metabolites, ceramide, ceramide-1-phosphate (C1P), and sphingosine-1-phosphate (S1P) are emerging as important signalling molecules that regulate cell growth, survival, immune cell trafficking, and vascular and epithelial integrity, and are particularly important in inflammation and cancer2,3.
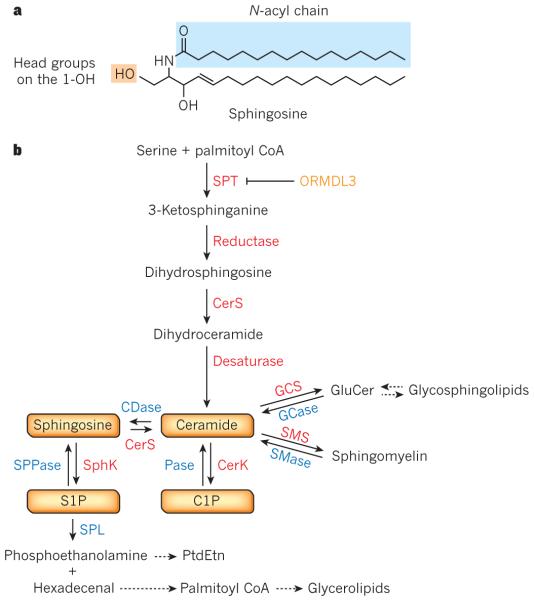
Figure 1. Sphingolipid metabolism and interconnected bioactive sphingolipid metabolites.
a, Structure of ceramide, with a sphingosine backbone. b, De novo sphingolipid biosynthesis starts with the condensation of palmitoyl coenzyme A (CoA) and serine by serine palmitoyltransferase (SPT), an enzyme that is negatively regulated by ORM1-like protein 3 (ORMDL3). This is followed by a series of reactions (catalysed by enzymes in red) leading to formation of ceramide and subsequent formation of sphingomyelin and glycosphingolipids. Ceramide can be metabolized to other bioactive sphingolipid species, phosphorylated by ceramide kinase (CERK) to ceramide-1-phosphate (C1P), or hydrolysed to sphingosine, which is then phosphorylated to sphingosine-1-phosphate (S1P) by sphingosine kinases (SphKs). S1P can be degraded by phosphatases to sphingosine or by the lyase (SPL) that cleaves it to phosphoethanolamine and hexadecenal, which are subsequently reincorporated into glycerolipid metabolic pathways. For simplicity, degradative enzymes (blue) for reutilization of sphingolipids in the salvage pathway are included but these reactions take place in different subcellular compartments (see Fig. 2). CDase, ceramidase; CerS, ceramide synthase; GCase, glucosylceramidase; GCS, glucosylceramide synthase; Pase, phosphatase; PtdEtn, phosphatidylethanolamine; SMase, sphingomyelinase; SMS, sphingomyelin synthase; SPPase, sphingosine phosphate phosphatase.
The past decade has seen an explosive advancement in the field of sphingolipid signalling based on the convergence of several key aspects. First, most of the regulatory proteins and enzymes involved in sphingolipid metabolism and the receptors for S1P have been cloned. This allowed the generation of knockout mice, yielding insights into the physiological functions of sphingolipid metabolites. Second, the advent of advanced mass spectroscopic techniques has brought the ‘omics’ revolution to sphingolipids, allowing for the simultaneous analysis and quantification of multiple species. Third, specific agonists and antagonists of S1P receptors and inhibitors of signalling enzymes were developed. The chief development among these was the discovery of FTY720 (fingolimod), a sphingosine analogue that alters immune cell trafficking and is already being used in the clinic for the treatment of multiple sclerosis4.
These are exciting times for the field and research continues apace. Several sphingolipid signalling protein structures have been solved, allowing for rational drug design. This Review will focus on the function of three key bioactive sphingolipids: ceramide, C1P and S1P, and their roles in inflammation. Although this is a normal physiological response to harmful stimuli such as infection, unchecked inflammation can lead to numerous pathophysiological states, including oedema, asthma, inflammatory bowel disease and associated cancer, and autoimmune disorders such as multiple sclerosis and rheumatoid arthritis. Sphingolipid metabolites play crucial parts at multiple stages of these disorders, and new mechanistic perspectives on their actions will be discussed. We will also highlight how knowledge gained in this relatively new field will aid in the development of therapeutic options for inflammatory disorders.
Sphingolipid metabolism
Sphingolipids are essential lipids consisting of a sphingoid backbone that is N-acylated with various fatty acids to form many ceramide species, which can have hundreds of distinct head groups. As this Review is mainly concerned with sphingolipid signalling and inflammatory diseases, we will focus on mammalian sphingolipids and their metabolism. Sphingolipid synthesis starts in the endoplasmic reticulum (ER) with the condensation of serine and palmitoyl coenzyme A (CoA) by serine palmitoyltransferase (SPT), the rate-limiting step, forming 3-ketosphinganine (Fig. 1b). SPT mutations can shift substrate preference to alanine, producing neurotoxic deoxysphingolipids that cause hereditary sensory and autonomic neuropathy type 1 (ref. 5). SPT activity is negatively regulated by ORMDL proteins6, and a genetic variant that increases ORMDL3 expression has been linked to increased risk of asthma7. Reduction of 3-ketosphinganine forms dihydrosphingosine that is then N-acylated by one of six ceramide synthases (CerS1–CerS6), each using specific acyl chains, typically with saturated or mono-unsaturated fatty acids with 14 to 26 carbons, forming dihydroceramides that are subsequently dehydrogenated to ceramides by dihydroceramide desaturase. Metabolism of ceramide to sphingomyelin and a vast array of complex glycosphingolipids mainly occurs in the Golgi (Fig. 2). ER to Golgi transport of ceramide is mediated by ceramide transfer protein (CERT) for sphingomyelin synthesis8, or by vesicular transport for glucosylceramide synthesis. Ceramides can also be phosphorylated in the Golgi by ceramide kinase (CERK) to form C1P, a rare species. Glycosphingolipid synthesis requires the transfer of glucosylceramide to the trans-Golgi network (TGN) by four-phosphate adaptor protein 2 (FAPP2), which also regulates vesicular trafficking from the Golgi to the plasma membrane9.
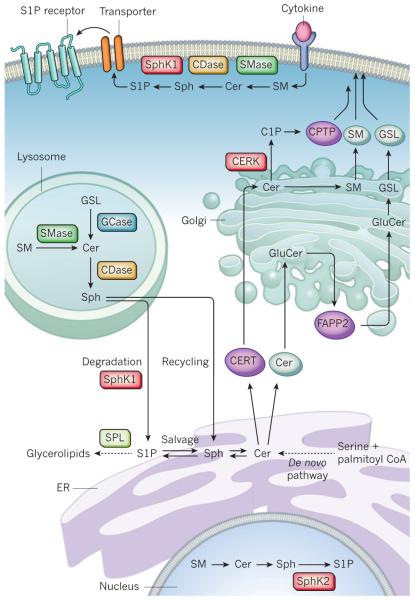
Figure 2. Subcellular compartmentalization of sphingolipid metabolism.
De novo ceramide (Cer) synthesis takes place in the endoplasmic reticulum (ER). Cer is delivered by ceramide transport protein (CERT) or vesicular transport to the Golgi for synthesis of ceramide-1-phosphate (C1P) (by ceramide kinase, CERK), sphingomyelin (SM), and glucosylceramide (GluCer). Four-phosphate adaptor protein 2 (FAPP2) then transports GluCer to the trans-Golgi for biosynthesis of complex glycosphingolipids (GSLs). SM and GSLs are delivered to the plasma membrane by vesicular transport and C1P by a C1P-specific transfer protein (CPTP). For signalling at the plasma membrane, sphingomyelinase (SMase), ceramidase (CDase), and sphingosine kinases (SphK) produce the bioactive metabolites Cer, sphingosine (Sph) and sphingosine-1-phosphate (S1P), respectively. S1P is then transported across the membrane. Membrane sphingolipids are internalized by the endocytic pathway and in the lysosome they are degraded by acidic forms of SMase, glycosidase (GCase) and CDase. The Sph formed can be metabolized to glycerolipids after phosphorylation by SphKs (probably SphK1) and cleavage by S1P lyase (SPL) or reutilized for sphingolipid synthesis in the salvage pathway. In the nucleus, SphK2-produced S1P inhibits histone deacetylases.
Sphingolipids have a rapid turnover and their levels are controlled by the balance between synthesis and degradation in multiple compartments2. Sphingolipids are degraded in lysosomes by glycosidases or acid sphingomyelinases that remove the head groups to form ceramides. Deacylation of ceramide by ceramidases is the only pathway known to generate sphingosine. Sphingosine can be recycled back to ceramide, and 50% or more of sphingosine molecules follow this reutilization pathway, which has a significant role in sphingolipid homeostasis2. Sphingosine can also be phosphorylated by one of two sphingosine kinases, SphK1 and SphK2, forming S1P, a pleiotropic bioactive metabolite and key intermediate in the sphingolipid-to-glycerolipid metabolic pathway10.
S1P can then either be dephosphorylated by phosphatases, including two S1P-specific, ER-localized phosphatases (SPP1 and SPP2), or irreversibly degraded by S1P lyase (SPL) to phosphoethanolamine and hexadecenal that can be incorporated into glycerolipids. The fatty aldehyde dehydrogenase ALDH3A2 converts hexadecenal to hexadecenoate, which is utilized for the formation of palmitoyl-CoA in glycerolipid synthesis, revealing the connection between sphingolipid and glycerolipid homeostasis11. ALDH3A2 is non-functional in Sjögren–Larsson syndrome, a disease characterized by ichthyosis and intellectual disability, suggesting that the accumulation of S1P degradation products contributes to the pathogenesis11.
The S1P gradient
S1P concentrations are high in blood and lymph, and low in tissues, presumably due to the higher activity of S1P degrading enzymes in tissue. The high level of blood S1P has important homeostatic functions in the maintenance of vascular integrity, and the S1P gradient is crucial for immune cell trafficking. S1P in blood is rapidly turned over12, suggesting robust mechanisms for its synthesis inside cells and its transport outside. Both SphK1 and SphK2 contribute to circulating pools of S1P. Erythrocytes13 and vascular endothelial cells14 are the main sources of blood S1P, and lymphatic endothelial cells are the main sources for lymph S1P15. S1P is exported out of vascular and lymphatic endothelial cells by the specific transporter Spns2, which is not present in platelets or erythrocytes, suggesting the existence of other transporters in these cells16,17. Although ceramide, which is also abundant in plasma18, is associated with very-low-density lipoprotein and low-density lipoprotein, S1P in plasma is bound to both albumin and apolipoprotein M (apoM), which preferentially associates with high-density lipoprotein. However, only high-density lipoprotein containing apoM–S1P is required to preserve endothelial barrier integrity19. ApoM produced by the liver influences plasma S1P levels by enhancing S1P biosynthesis for secretion by hepatocytes, demonstrating that the liver is involved in S1P dynamics and that apoM delivers S1P to extrahepatic tissues20,21.
Sphingolipid signalling
Multiple stimuli affect sphingolipid biosynthesis and metabolism by regulating key enzymes in a spatial and temporal manner, leading to the restricted production of ceramide, C1P and S1P2. For example, pathogens, oxidative stress and cytokines activate acid or neutral sphingomyelinases to generate ceramide in specific compartments2. Sphingosine generated by ceramidase is another sphingolipid metabolite involved in cell signalling22 (see Review by Platt on page 68). However, its role in inflammatory responses is not yet clear23,24. Some inflammatory mediators also activate SphK1 by extracellular signal regulated kinase (ERK)-mediated phosphorylation, which promotes SphK1’s translocation to the plasma membrane, at which its substrate, sphingosine, is localized and/or generated, resulting in transient elevations in S1P levels3,25. Although these studies were mainly carried out with cultured cells, the development of high-sensitivity mass spectrometry methods has led to demonstrations of changes in levels of bioactive sphingolipids being reported in specific tissues from mouse inflammatory disease models26-29 and, in a few cases, in human samples30,31. More studies are needed to rigorously correlate bioactive lipids with disease stage. Understanding what bioactive sphingolipids do and how they signal is crucial for our understanding of their functions in inflammatory responses.
Ceramide
Despite the involvement of ceramide in numerous biological processes, only a few of its direct targets have been described. The best examples are ceramide-activated serine/threonine protein phosphatases, including PP1, PP2A and PP2C32,33. PP2A is found in complex with SET (also known as I2PP2A), which inhibits PP2A function. Ceramide binds to SET, relieving its inhibitory actions33. Activation of PP2A that leads to dephosphorylation of Akt, a potent promoter of cell survival, could partly explain the pro-apoptotic actions of ceramide. In additional, PP1 could be the target of ceramide generated at the plasma membrane32. Stimulation of the atypical protein kinase PKCζ by ceramide, probably concomitant with its membrane recruitment, also leads to inhibition of Akt34.
Another concept that has been subject to renewed interest is the formation of ceramide-enriched membrane microdomains or platforms as a mechanism by which ceramide transduces intracellular signalling. Alterations in membrane domains, owing to the elevation of ceramide and its tendency to self-associate, influence membrane composition and interactions of lipids or signalling proteins. These ceramide-rich platforms have been implicated in a variety of signalling cascades in immune cells, including activation of B cells, bacterial pathogen infections and release of cytokines during infection; they are also especially important in the induction of apoptosis35.
C1P
C1P is formed on the TGN by CERK. Here, C1P activates cytosolic phospholipase-A2α (cPLA2α)36, the enzyme that releases the eicosanoid precursor arachidonate. Although early concerns regarding cPLA2α activation by C1P were raised37, others have shown that not only does C1P bind cPLA2α through an RxRH motif in its amino-terminal CaLB (also known as C2 lipid-binding domain) and recruit it to the membrane at which its phospholipid substrates reside, C1P also stimulates its activity and therefore has been implicated in eicosanoid production36,38. Conversely, C1P has been shown to inhibit ADAM17 (also known as tumour necrosis factor (TNF)-converting enzyme, or TACE), the major metalloprotease responsible for cleaving pro-TNF to release the active inflammatory form, probably by binding to one or more similar tribasic motifs39.
S1P
The many diverse roles of S1P in innate and adaptive immunity, including immunosurveillance, immune cell trafficking and differentiation, immune responses and endothelial barrier integrity are mediated by its binding to one of five G-protein-coupled receptors, named S1PR1– S1PR5 (refs 3, 40, 41). Downstream signalling of these receptors is complex as they are differentially expressed in immune and endothelial cells and couple to a varied set of heterotrimeric G proteins. For example, activation of S1PR1 generally promotes cell migration and the egress of T and B lymphocytes from lymphoid tissues42, whereas S1PR2 inhibits motility to promote retention of B cells in germinal centres43. S1P produced inside cells by activation of SphK1 can be secreted by SPNS2 or promiscuous ABC transporters16,17,44. S1P, in turn, signals through its receptors in a paracrine and/or autocrine manner, termed ‘inside-out’ signalling. However, the crystal structure of S1PR1 suggests that S1P must slide laterally within the plane of the bilayer to access the binding pocket45, raising the question of whether S1P needs to be secreted for its autocrine actions.
Much less is known about the intracellular targets of S1P. Within the past decade, new intracellular targets of S1P have been characterized. S1P formed by SphK1 in response to TNF or interleukin-1 (IL-1) binds to TNF receptor-associated factor 2 (TRAF2) and cellular inhibitor of apoptosis 2 (cIAP2), respectively, and enhances their lysine-63-linked polyubiquitylation activities46,47. In response to IL-1, which has a pivotal role in autoinflammatory diseases, cIAP2 and SphK1 form a complex with interferon regulatory factor-1 (IRF1), leading to its polyubiquitylation and activation. Consequently, IRF1 enhances expression of the chemokines CXCL10 and CCL5 that recruit mononuclear cells to sites of sterile inflammation47. Thus, in addition to canonical S1P receptor signalling, these new intracellular functions of S1P might be involved in the immune and inflammatory responses of these potent cytokines. Furthermore, S1P in the nucleus, formed by SphK2 or by inhibition of SPL, binds and inhibits the histone deacetylases HDAC1 and HDAC2, linking sphingolipid metabolism to inflammatory and metabolic gene expression48-50. Further studies are needed to solidify these new concepts.
Sphingolipids in endothelial biology and function
The vascular endothelium forms a barrier that lines blood vessels and plays an important part in cardiovascular homeostasis and blood flow, and in controlling the passage of leukocytes into and out of the bloodstream. Ceramide and S1P have opposing effects on vascular endothelium functions: whereas increased ceramide leads to endothelial barrier dysfunction51, S1P is crucial for maintenance of vascular integrity52-54 (Fig. 3a).
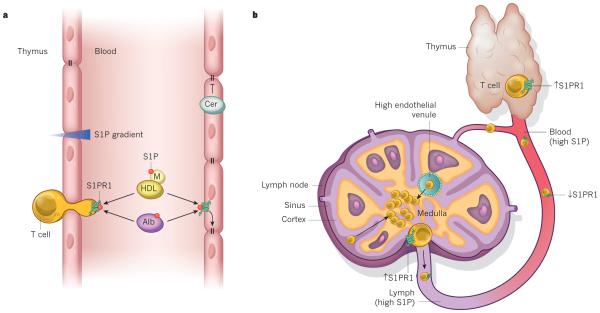
Figure 3. S1P and S1PR1 control lymphocyte trafficking and vascular integrity.
a, The sphingosine-1-phosphate (S1P) level in lymphoid tissues, such as the thymus, is low compared with the blood, forming an S1P gradient (shaded red) that attracts lymphocytes and promotes S1PR1-dependent egress into the blood. In the blood, S1P is bound to albumin (Alb) and apolipoprotein M (M) in high-density lipoprotein (HDL), and signalling by S1PR1 maintains endothelium barrier function by promoting cell–cell interactions (right). Conversely, ceramide (Cer) can promote barrier leakage. b, When T cells are ready to exit the thymus and enter the blood, S1PR1 is re-expressed so that T cells can respond to the chemotactic effect of high S1P levels in circulation. When T cells re-enter the blood, S1P downregulates S1PR1. Signals that control lymphocyte entry through high endothelial venules and retention in lymph nodes are not shown. Lymph node egress into lymphatic vessels also requires S1PR1. Increased lymphoid tissue S1P, by SPL inhibition, or in the presence of S1PR1 modulators such as FTY720, blocks egress of lymphocytes by disruption of the S1P gradient or desensitization of S1PR1 on T cells, respectively.
Pro-inflammatory cytokines, oxidative stress and injurious insults, such as platelet activating factor (PAF), TNF and lipopolysaccharide, generate excess ceramide in endothelial cells linked to increased vascular permeability and dysfunction (Fig. 3a). Generation of ceramide is both stimulus- and context-specific, and is due to the activation of acid or neutral sphingomyelinase, or increased de novo biosynthesis. Ceramide triggers several pathways that induce endothelial cell death, including activation of caspases, or PP1 or PP2A2,55, and increasing mitochondrial permeability by forming ceramide-enriched platforms capable of translocating proteins. Moreover, PAF-induced formation of ceramide microdomains drives endothelial nitric oxide synthase (eNOS) activation and contributes to barrier dysfunction56. Ceramides have also been linked to growth arrest, cytoskeleton rearrangements, oxidative stress and senescence of endothelial cells2. Thus, ceramides regulate important endothelial cell functions that are thought to be responsible for the pathogenesis associated with vascular dysfunctions, including emphysema, sepsis and acute respiratory distress syndrome.
Using animal models of acute and chronic inflammation, it has been convincingly demonstrated that plasma S1P limits disruption of vascular endothelial monolayers and reduces oedema57 (Fig. 3a).
S1P activates endothelial S1PR1, leading to enhanced Rac-dependent cytoskeleton rearrangements, contacts between cells and the matrix, adherens junction assembly and barrier integrity3,41.
Lymphocytes circulate through lymph nodes for immune surveillance, entering at high endothelial venules (HEVs) — specialized blood vessels. Until recently, it was not known how HEVs allow lymphocyte transmigration, which increases during immune responses, and maintain vascular integrity. A study demonstrated that podoplanin expressed on HEV fibroblastic reticular cells binds and activates platelet C-type lectin-like receptor-2 (CLEC2)58. Activation of CLEC2 on extravasated platelets leads to the release of S1P in the perivenular space of HEVs. S1P, in turn, enhances vascular endothelial (VE)-cadherin expression for maintenance of the integrity of HEVs during immune responses58. It is likely that the S1P–S1PR1 axis is also involved in regulating the integrity of HEVs.
During inflammation, infiltrating immune cells and the production of pro-inflammatory cytokines increase endothelial permeability. S1P signalling attenuates the increased permeability induced by the inflammatory mediators histamine and PAF57. It is still not clear whether levels of S1P in plasma are reduced in systemic inflammation or sepsis. However, because levels of the S1P carrier protein apoM are decreased in patients with sepsis and systemic inflammatory response syndrome59, it is possible that S1P plasma levels are reduced in these conditions and thus contribute to vascular leakage.
Although S1PR2 does not regulate basal permeability, it protects against acute vascular barrier disruption and lethality after antigen challenge or PAF administration60. Endothelial S1PR2 inhibits Akt activation, suppressing eNOS and NO generation, and protects against the disassembly of adherens junctions by decreasing S-nitrosylation of β-catenin and thereby vascular leakage41,60. By contrast, during endotoxaemia, endothelial S1PR2 enhances vascular permeability and expression of pro-inflammatory and pro-coagulation molecules by activating Rho and p38 SAPK signalling pathways61. Together, these observations suggest that S1PR1 and S1PR2 have specific roles in preventing or triggering vascular leakage, probably by activation of distinct signalling pathways. For example, S1PR1 mediates Gi-dependent Rac and Akt activation, whereas S1PR2 inhibits them and stimulates Rho3,41. Alternatively, the S1P receptors might be temporally and spatially localized and upregulated during inflammation.
An unexpected function of endothelial S1PR1 was discovered using S1PR1-specific agonists that suppressed the cytokine storm and recruitment of innate immune cells to the lung during influenza infection62. The efficacy of S1PR1 agonists was a result of the inhibition of common pathways downstream of multiple innate sensing pathways that are important for interferon production63. A better understanding of how S1P receptors and their downstream signalling are regulated and how they affect the vascular system could provide the basis for developing improved therapies for vascular disorders.
Sphingolipids and inflammation
During inflammation, innate and adaptive immune cells enter sites of infection or injury and the activation of cytokine networks that follows helps to protect the host. Sphingolipid metabolites have key roles in the regulation of both trafficking and functions of immune cells42,64, and there are indications that sphingolipid metabolism and S1P receptors might be altered by inflammation.
S1P drives the differentiation of many types of immune cells, inducing changes in their functional phenotypes and regulating production of pro-inflammatory cytokines and eicosanoids. These topics will not be covered here as they have recently been extensively discussed (see refs 3, 42, 64 and 65 for authoritative reviews). In particular, S1P has emerged as a central regulator of lymphocyte egress. Although the S1P–S1PR1 axis regulates trafficking and migration of most types of immune cells (such as T and B lymphocytes, haematopoietic progenitors, macrophages, dendritic cells, neutrophils, mast cells, natural killer T cells and osteoclasts3,42), the regulation of T- and B-lymphocyte trafficking is the best understood and will be used to illustrate the primary mechanisms involved.
S1P and lymphocyte trafficking
S1P concentrations in blood and lymph are much greater than in tissues, and lymphocyte egress from the thymus or secondary lymphoid tissues is dependent on the cells sensing this gradient through activating S1PR1 (ref. 42). When ready to leave the thymus, T lymphocytes upregulate S1PR1 (ref. 66) and exit in response to the S1P gradient (Fig. 3b). S1PR1-dependent Rac activation is required for trans-endothelial migration of T cells and lymph node egress67. After entering the blood, high S1P levels desensitize S1PR1, owing to phosphorylation by guanine nucleotide-binding protein coupled receptor kinase-2 (GRK2)68. Coordinated action of selectins, integrins and especially the retention signals of the CCL21 chemokine receptor CCR7 are required for lymphocytes to enter the lymph nodes through HEVs42. T lymphocytes sample the environment randomly — cortical sinus probing is followed by S1PR1-dependent entry69 and then flow into the efferent lymph containing high S1P15 (Fig. 4b). During inflammation, lymphocyte retention is increased by the loss of S1PR1 expression, its internalization and degradation. After several rounds of division, newly generated effector cells upregulate S1PR1, lose the CCR7 retention signal and then exit into circulation and travel to locations of injury and inflammation42. The immune system co-opts the S1P–S1PR1 axis to determine the establishment of resident memory T cells, a distinct lineage that are embedded in non-lymphoid tissues and that mediate protective immunity. Regulation of the transcription factor KLF2 and its target gene S1pr1 in mice by cytokines determines whether CD8+ T cells re-enter circulation or become tissue resident70.
Figure 4. Ceramide at the nexus of obesity and inflammation.
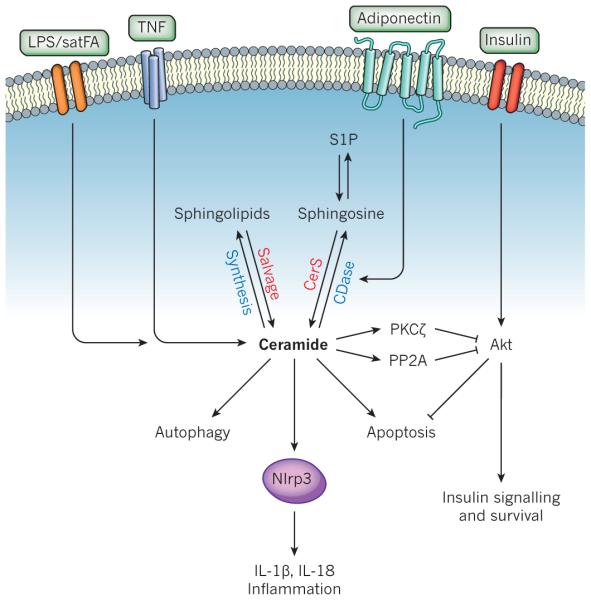
Ceramide is generated in response to obesity signals (such as saturated fatty acids, satFA), lipopolysaccharide (LPS) or pro-inflammatory cytokines (such as TNF) by enhancement of de novo biosynthesis or sphingolipid recycling. Ceramide can then signal to a variety of pathways that promote inflammation and the deleterious effects of obesity. Ceramide can bind to and inhibit SET/I2PP2A (not shown), leading to the activation of PP2A, which dephosphorylates and inhibits Akt, blocking many of the actions of insulin signalling and leading to insulin resistance, and decreased cell survival; activate PKCζ, also leading to the inhibition of Akt; induce autophagy, which can influence innate immune responses; activate the Nlrp3 inflammasome to promote the processing and secretion of the pro-inflammatory cytokines IL-1β and IL-18; and induce apoptosis. Binding of adiponectin to its receptors stimulates ceramidase (CDase), degrading ceramide to sphingosine, which is then phosphorylated to sphingosine-1-phosphate (S1P). Insulin stimulates Akt. It should be noted that this diagram is not meant to imply that all of these events occur in the same cell. CerS, ceramide synthase.
In addition to regulating the egress of B cells, the S1P–S1PR1 axis plays a part in the positioning of marginal-zone B cells in the spleen71. These B cells are the first line of defence against systemic blood-borne antigens. Levels of S1P are high in the splenic marginal zone, causing desensitization of S1PR1 on resident B cells that allows them to migrate into the follicles in response to the follicular-dendritic-cell-produced chemokine CXC13 (ref. 42). B cells in the marginal zone shuttle constantly between this zone and follicles, depending on the S1P gradient. This allows for the rapid delivery of antigens from the blood to the follicles71. However, in germinal centres in which mature B cells proliferate, differentiate and switch antibody class production, S1PR2 activation and its downstream effectors Gα12, Gα13 and p115RhoGEF counteract signalling by Akt, decreasing survival and responses to follicular chemoattractants, thus helping to focus the cells to the follicle centre43. It remains to be seen whether this gradient of S1P exists between the germinal centre and the follicle boundary. The remaining major challenges are to understand how this gradient is produced and how to measure S1P pools in local environments. For S1P to be sensed during egress, its levels in tissue interstitial fluid would be expected to be very low to ensure that B-cell S1PR1 could respond to higher concentrations at the luminal surfaces of egress sites. However, although interstitial levels are generally assumed to be due to high S1P degrading activity in tissues, so far it has not been possible to directly measure interstitial fluid S1P levels. It is anticipated that this will be accomplished soon with the improved sensitivity of newer mass spectrometers.
S1P could be pro- or anti-inflammatory
The inflammatory role of S1P formed in immune cells by SphK1 or SphK2 is still unclear. This conundrum began when inflammatory responses were examined in Sphk1and Sphk2 knockout mice. Whereas some studies have reported that colonic inflammation26 and synovial inflammation in TNF-induced arthritis72 are reduced in Sphk1 knockout mice, others have reported that these mice have normal acute and chronic inflammatory responses73. The retraction of a paper that suggested SphK1 had a role in regulating pro-inflammatory responses in a mouse sepsis model was concerning74, and there is no evidence that inhibiting SphK1 or deleting its gene will reduce sepsis. Although Sphk2 knockout mice have normal neutrophil functions, in a model of bacterial lung infection these mice have accelerated disease progression75. Similarly, neuroinflammation76 and lung inflammatory injury77 induced by lipopolysaccharide were increased by deletion of Sphk1.
It is possible that S1P formed by SphK1 or SphK2 has distinct functions and responses to stimuli in specific types of cells and tissues. This could also depend on the expression of S1P receptors and their specific downstream targets. Also, whereas deletion of Sphk1 decreases S1P levels in blood, deletion of Sphk2 increases it26,78, probably due to upregulation of Sphk1 expression and an increase of S1P in some tissues but not others78. Changes in levels of S1P in the circulation and in tissues could affect immune-cell differentiation and functional phenotypes64. Production of S1P is spatially and temporally regulated and its physiological and inflammatory outcomes are context-, tissue- and source-dependent. Future studies with conditional knockout mice could clarify the inflammatory roles of S1P and the kinases that produce it in specific tissues.
Sphingolipids may mediate inflammatory signalling by TNF
TNF, a key pro-inflammatory cytokine and therapeutic target in inflammatory diseases, stimulates sphingolipid metabolic enzymes including sphingomyelinases, ceramidase and SphK1 (refs 2, 79). Several sphingolipid metabolites have been suggested to be involved in TNF signalling and chemokine production based on studies of reduction or overexpression of these enzymes. For example, cells that are deficient in acid sphingomyelinase or acid ceramidase have decreased production of CCL5 induced by TNF, possibly due to regulation of sphingosine levels23, whereas knockdown of the gene encoding neutral sphingomyelinase regulates adhesion molecules independently of NF-κB activation79. The effects of deletion or knockdown of SphK1 have yielded conflicting results. Initially, overexpression of a dominant-negative SphK1 mutant80 or SphK1 downregulation81,82 reduced NF-κB activation, production of cytokines and chemokines, and induction of COX2 and PGE2 production in response to TNF. More recently, S1P formed by TNF-mediated activation of SphK1 was suggested to be crucial for TRAF2-mediated K63 polyubiquitylation of RIP1, a key step in NF-κB activation46. However, studies have now found that downregulation of SphK1 had no effect on TNF-induced NF-κB activation but enhanced CCL5 expression through the p38 MAPK pathway, whereas downregulation of SphK2 reduced CCL5 expression without affecting NF-κB83. Moreover, TNF-mediated NF-κB activation and cytokine expression in murine macrophages that lack both SphK1 and SphK2 were not altered but these cells had increased sphingosine and ceramide levels and autophagy24. Clearly, resolving these discrepancies requires further study; however, there are several intriguing possibilities. The interconversion of sphingolipid metabolites is rapid, and fluxes of sphingolipids depend on the cell type; thus, distinct bioactive sphingolipids could be increased by TNF and their targets could be cell-type dependent. The outcome of inside-out signalling by S1P induced by TNF, as observed in certain cell types3, would depend on the expression of S1P receptors and their downstream effectors. It is also possible that deletion of sphingosine kinases induces compensatory mechanisms. Moreover, TNF-induced inflammatory responses are not only regulated by NF-κB but are also controlled by crosstalk between NF-κB with other context–dependent pathways. Finally, TNF signalling leading to NF-κB and cytokine and chemokine production is now appreciated to be more complex than was originally suggested84. The recent discovery of RIP1-dependent and -independent activation of the early and late phases of IκB kinase (IKK), which are differentially regulated by low and high doses of TNF that lead to the expression of distinct sets of NF-κB target genes84, suggests a potential explanation for the divergent findings on the role of SphK1 and S1P. Exploring these possibilities might reconcile contradictory observations and shed new light on the inflammatory roles of S1P.
Ceramides and inflammation
During host–pathogen defence, pattern recognition receptors, including Toll-like receptors (TLRs) and Nod-like receptors (NLRs), expressed by sentinel cells, macrophages and dendritic cells, bind microbial products to initiate innate immune responses and prime adaptive immunity. Integration of data from sphingolipidomics and transcriptomics of macrophages treated with lipopolysaccharide, the ligand for TLR4, revealed that several enzymes in sphingolipid metabolism and their products, particularly ceramide, were coordinately elevated85,86. Several intriguing ideas have been proposed to explain how ceramide links TLR4 and other members of this family to inflammation (Fig. 4). In macrophages, increased de novo ceramide biosynthesis is required for autophagosome formation86, which is thought to have key roles in innate immunity87. A question still to be answered is whether changes in autophagy owing to ceramide contributes to immunity and/or inflammation. Disruption of membrane microdomains in Cers2 knockout mice, which are unable to make very-long-chain ceramides, prevented TNF-R1 internalization and downstream signalling88.
Another area that has received much attention is the connection between low-grade chronic inflammation and the pathogenesis of obesity and metabolic dysfunction89. A recently uncovered mechanistic link between increased inflammation and obesity is the activation of TLR4 by saturated fatty acids, leading to transcriptional activation of ceramide biosynthetic genes, including those that encode SPT and specific CerS isoforms, in an IKK-β-dependent manner29. Interestingly, although not required for TLR4-dependent inflammatory cytokine induction, elevated ceramide production was required for TLR4-dependent insulin resistance, owing to inhibition of Akt29, the activation of which is necessary for stimulated glucose transport90 (Fig. 4). Likewise, CerS2 knockout mice are glucose intolerant, owing to the inability of the insulin receptor to translocate to the disrupted membrane platforms, preventing Akt activation91. Together, these studies implicate ceramide as a mediator linking lipid-induced inflammatory pathways and insulin resistance.
The NLR family member NLRP3 inflammasome is activated by diverse non-microbial danger-associated molecular patterns (DAMPs) derived from damaged cells, and induces inflammation by increasing IL-1β and IL-18 secretion. In obese mice, lipotoxicity-associated ceramide was shown to increase activated caspase-1 in a Nlrp3 inflammasome-dependent manner in macrophages and adipose tissue92. This is the first suggestion that the Nlrp3 inflammasome senses increases in intracellular ceramide (Fig. 4)92,93. Moreover, age-related increased thymic ceramides also leads to Nlrp3 inflammasome-dependent caspase-1 activation94, suggesting that this form of ceramide elevation is responsible for the collapse of the thymic stromal cell microenvironment and subsequent decreased production of naive T cells and lower immune surveillance in the elderly94.
Ceramides and adipokines
An intriguing potential link has surfaced between sphingolipid metabolism and the pleiotropic actions of the adipocyte hormone adiponectin. By binding to its receptors AdipoR1 or AdipoR2 adiponectin decreases inflammation and increases insulin sensitivity95. AdipoR1- and AdipoR2-stimulated ceramidase degraded ceramide to sphingosine, which was converted into the pro-survival S1P; evidence indicates that ceramidase activation initiates many adiponectin actions (Fig. 3)28. It is unclear whether AdipoR1 and AdipoR2 have intrinsic ceramidase activity, although a recent report suggests that AdipoR1, but not AdipoR2, induces caveolin-dependent recruitment of neutral ceramidase96.
Subsequent studies demonstrated that the adiponectin–ceramide axis is also involved in fibroblast growth factor-21 (FGF21)-mediated regulation of carbohydrate and lipid metabolism, and energy utilization. The beneficial effects of FGF21 were abolished in adiponectin-null mice, which were resistant to FGF21-induced decreases in ceramide and increases in energy expenditure97. Adiponectin also mediated the FGF21-induced decrease in production of liver pro-inflammatory cytokines in obese, diabetic mice98. Experiments are needed to determine which adiponectin-mediated effects depend mainly on decreasing ceramide signalling and which depend on increasing S1P signalling. Interestingly, thiazolidinediones — agonists for the nuclear receptor PPAR-γ, widely used for management of type 2 diabetes — also induce FGF21 and adiponectin, and decrease ceramide in plasma and the liver97. These FGF21 and thiazolidinedione effects bring ceramides into the spotlight for research on new therapeutics for obesity-induced inflammation and metabolic disorders.
New insights on the role of C1P in inflammation
C1P is a relatively new addition to the group of bioactive sphingolipid metabolites involved in inflammation. C1P activates cPLA2α36, which releases arachidonic acid in the rate-determining step in eicosanoid production. As eicosanoids drive the pathogenesis of many inflammatory disorders, understanding the role of C1P in this process is crucial. An intriguing example of the latter was the characterization of a unique C1P-specific transfer protein called CPTP38. Depletion of CPTP, which is present in the cytosol and associated with the TGN and plasma membrane, increases C1P in the Golgi and nucleus, but decreases it in the plasma membrane. This suggested that CPTP controls levels of C1P produced by CERK in the Golgi by transferring it to the plasma membrane, thereby suppressing cPLA2α activity and reducing arachidonic acid release and eicosanoid production38. The C1P that CPTP transfers to the plasma membrane is probably rapidly degraded by lipid phosphatases to ceramide. Another intriguing observation from this study was that changes in C1P levels directly correlated with changes in sphingosine and S1P, raising the possibility that C1P might be a precursor of S1P that is mediated by, yet to be characterized, deacylase activity38. Global CERK inactivation has only modest effects on inflammatory responses, in contrast to cPLA2 knockout, and further studies are necessary to address this discrepancy. Nevertheless, acute downregulation of CERK using short interfering RNA did reduce eicosanoid production in a mouse model of airway hyper-responsiveness99.
The concept of immunomodulation by C1P has become increas- ingly intricate as other studies have shown that C1P also negatively regulates inflammatory cytokine production. C1P produced by CERK regulates TNF processing and secretion by direct inhibition of TACE39. In addition, exogenous C1P was found to suppress lipopolysaccharide mediated production of TNF, IL-6, IL-8 and IL-1β in human peripheral blood mononuclear cells100. Although exogenous C1P can stimulate macrophage migration by a pertussis-toxin-sensitive GPCR101, a cell surface C1P receptor has never been identified. It is also unclear what biological effects, if any, are regulated by circulating C1P.
Therapeutic interventions in inflammatory disorders
Much of the clinical interest in sphingolipid metabolites and their signalling was spurred by the discovery of the mechanism of action of the immunosuppressant drug FTY720 (ref. 102). In vivo, SphK2 is mainly responsible for the phosphorylation of FTY720 to FTY720-P, an S1P mimetic and agonist for all S1P receptors except S1PR2. Binding of FTY720-P to S1PR1 leads to its downregulation, thereby effectively acting as a functional S1PR1 antagonist. Down-modulation of S1PR1 induces the retention of lymphocytes in lymphoid organs, termed lymphopenia, because the receptor is required for their exit into the blood up the S1P gradient; this prevents adaptive immune surveillance of tissues, and thus can ameliorate autoimmune diseases.
Autoimmune diseases
Thousands of patients have been treated with FTY720 for the relapsing-remitting form of multiple sclerosis. In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, FTY720 prevented pathogenic T cells committed to T helper (TH) 1 and TH17 lineages from crossing the blood–brain barrier into the central nervous system4. Down-modulation of S1PR1 inorectal cancer astrocytes by FTY720 reduced astrogliosis103, a hallmark of multiple sclerosis, and promoted remyelination and recovery of nerve conduction. These are likely explanations for the significant decrease in inflammatory markers and brain atrophy after FTY720 treatment of patients with multiple sclerosis4.
FTY720 and other next-generation S1PR1-modulating drugs are now in various stages of clinical trials for the treatment of a variety of immune diseases. One of the side effects of these modulating drugs is the transient reduction of heart rate by the activation of GIRK potassium channels downstream of cardiomyocyte S1PR1 (ref. 104), but with careful monitoring of patients after the first dose this should not prevent their use. FTY720 also induces pulmonary vascular leakage in mice by inducing degradation of S1PR1 (ref. 105). Receptor pharmacology predicts that competitive S1PR1 antagonists should not have this adverse effect; however, they could reduce barrier function, owing to S1PR1 blockade. So far, S1PR1 antagonists that are effective in various disease models, including EAE, have not been tested in the clinic. Even with FTY720’s efficacy, the fact that it is well tolerated in patients and the convenience of oral administration, improvements could still be made. A deeper knowledge of S1P and its receptors, and an understanding of the beneficial and adverse side effects of targeting S1P receptors could lead to the development of even better treatments.
Rheumatoid arthritis
Rheumatoid arthritis is an autoimmune disease characterized by inflammation in movable joints. High levels of S1P have been found in the synovial fluid of patients with rheumatoid arthritis30; however, conflicting data were reported in different mouse rheumatoid arthritis models regarding the involvement of SphK1. Although Sphk1knock-out mice showed normal responses in models of collagen-induced arthritis73, synovial inflammation in TNF-induced arthritis was reduced72. Hence, this area of research needs to be re-examined.
Lymphopenia-inducing drugs such as FTY720 have potential as rheumatoid arthritis therapeutics, FTY720 was protective in a mouse model of rheumatoid arthritis106; however, there are no current clinical trials. But, phase II clinical trials are ongoing to target S1P signalling by a completely different mode based on the finding that inhibition of SPL with the food dye THI (2-acetyl-4-tetrahy-droxybutylimidazole) induces lymphopenia by elevating the levels of S1P in lymphoid tissues, disrupting the S1P gradient lymphocytes use for egress107. Subsequent studies of genetically deficient SPL mice revealed that they also had a disrupted S1P gradient and lymphope- nia. On the basis of THI structure, a second compound, LX2931, was developed that was effective in a mouse model of rheumatoid arthritis and is now in phase II trials108. It remains to be seen whether direct inhibitors of SPL are clinically useful.
Inflammatory bowel disease
Inflammatory bowel disease (IBD), a result of chronic inflammation of the gastrointestinal tract, is due to abnormal host immune responses to the intestinal microbiome109. Millions of people world-wide have ulcerative colitis and Crohn’s disease, the major types of IBD. Studies using common acute and chronic epithelial injury colitis models have underscored a role for the SphK1–S1P–S1PR1 axis in IBD. Knockout of the gene encoding SphK1 or specific SphK1 inhibitors greatly reduce colitis severity, cytokine production and systemic inflammation26,78. In agreement, S1P is elevated during colitis and SphK1 expression is increased in patients with ulcerative colitis26. Several studies have also shown that FTY720 and other pharmacological agents that target S1PR1 are very effective in rodent models of colitis78,110,111, and several second-generation S1PR1 modulators are currently in clinical trials for patients with ulcerative colitis.
Persistent inflammation of the colon increases the risk of developing colorectal cancer112. There is increasing evidence from mouse models that SphK1 and S1P may be a link between chronic intestinal inflammation and the development of colitis-associated cancer (CAC), and that both are important for colon cancer development and progression78,113,114. An intracellular role for SphK1 in intestinal adenoma cell proliferation and polyp size was shown by deletion of SphK1 in ApcMin/+mice, a model of intestinal neoplasia113. Upregulation of SphK1 during colitis and CAC in mice leads to NF-κB-dependent production of IL-6, robust activation of the transcription factor Stat3, and subsequent upregulation of its target gene, S1pr1. FTY720 impeded the SphK1–S1P–S1PR1 axis, suppressing the NF-κB–IL-6–Stat3 vicious feed-forward amplification loop and CAC78. Thus, it seems that FTY720 and second-generation S1PR1 modulators should be considered for the treatment of inflammation-driven cancers.
Asthma
Sphingolipid metabolites contribute to the pathogenesis of asthma, an increasingly prevalent chronic airway inflammation characterized by intermittent airflow obstruction and increased mucus production. S1P levels are elevated in the lungs of patients with allergic asthma. In mice, S1P is involved at multiple stages of the asthmatic response: it promotes the contraction of airway smooth muscle cells, the induction of airway hyper-reactivity by S1PR1 and/or S1PR3 (refs 115, 116), and regulates activation and functions of mast cells, eosinophils and dendritic cells27,117. Inhalation of an SphK1 inhibitor, or FTY720, mitigated asthma in rodent models27,117. De novo ceramide synthesis has also been implicated in human asthma based on the association of a 17q21 single nucleotide polymorphism (SNP) with increased expression of ORMDL3 (ref. 7), a negative regulator of SPT, the first enzyme in sphingolipid synthesis6,118. Patients with this SNP are at increased risk of developing childhood asthma as well as virus-induced respiratory wheezing119. However, how ORMDL3 is involved in asthma remains a matter of debate. It has been shown that allergens upregulate ORMDL3 expression, and overexpression of ORMDL3 increased expression of asthma-associated chemokines and metalloproteases and the unfolded protein response (UPR), suggesting that ORMDL3 and asthma may be linked through the UPR pathway120. ORMDL3 also promotes eosinophil trafficking, recruitment and activation121. By contrast, although ceramide is elevated in murine models of asthma122, decreased de novo sphingolipid biosynthesis enhances bronchial reactivity by affecting intracellular magnesium homeostasis in the absence of inflammation123. Although these studies functionally link de novo sphingolipid biosynthesis to asthma, many questions remain unanswered, presenting challenging opportunities for research.
Future perspectives
The study of bioactive sphingolipids in inflammation is only now coming to the foreground. As always in science, answering one question leads to many more, and this is especially true of these enigmatic sphingolipids. Clearly, more work is required to understand how inflammation regulates bioactive sphingolipid levels, the activities of the enzymes that control their levels and the proteins they signal through, and how this shapes the immune response. Although around a dozen extracellular and intracellular targets of signalling sphingolipids have been identified, it is unlikely that we have found them all, based on their pleiotropic effects. This is especially important for understanding the functions of ceramide in inflammation as only a few direct targets have been confirmed and how it regulates all inflammasomes remains a mystery. The complexity of sphingolipid metabolism and the ‘sphingolipidome’, and the fast interconversion of bioactive sphingolipids make this task even more daunting. This endeavour goes hand-in-hand with current systems-biology research, and it will be greatly aided by advanced sphingolipidomics — which can quantitatively measure changes in sphingolipids bound to their protein targets — combined with the development of conditional and tissue-specific sphingolipid-metabolic-enzyme knockout mice. Moreover, current sphingolipidomic technology for simultaneous quantification during inflammation of multiple sphingolipid species, for example, ceramides with different fatty acids, will be particularly helpful as these signalling molecules could have distinct and, sometimes, even opposing functions. Such a diverse array of bioactive sphingolipids could provide immune cells with a large toolkit to fine-tune specific inflammatory responses. Finally, improvement of matrix-assisted laser desorption ionization (MALDI) technology may, in the future, allow imaging of the S1P gradient in tissues such as the lymph nodes, the thymus and the spleen; this is crucial for our understanding of the role of the S1P– S1PR1 axis in lymphocyte trafficking.
Given the success of FTY720 for treatment of multiple sclerosis, it is hoped that next-generation S1PR1 modulators will find even wider therapeutic uses in other inflammatory disorders — as has been suggested by pre-clinical data. Progress in targeting other S1P receptors will increase our basic knowledge of their functions and how S1P can induce pro- and anti-inflammatory responses in different cell types, and will enable the rational design of more selective and potent inhibitors of immune responses. There are ongoing clinical trials with other interventions that target bioactive sphingolipids, such as Sphingomab, a monoclonal antibody that neutralizes S1P; ABC294640, which inhibits SphK2; and amitriptyline, which inhibits acid sphingomyelinase, and perhaps they will turn out to be useful for inflammatory disorders. Although such advances for other sphingolipid metabolites are still to be made, now that X-ray crystal structures of sphingolipid metabolic enzymes are available and carriers and targets of bioactive metabolites are gradually being elucidated, it is expected that drugs will be developed to interfere with specific sphingolipid-mediated functions, which are important for the pathogenesis of human inflammatory disease. ■
Acknowledgments
We thank S. Milstien for critically reading the manuscript. This work was supported by grants R37GM043880, R01AI500941 and R01CA61774 from the US National Institutes of Health. We apologize to those whose work was not cited owing to space constraints.
Footnotes
The authors declare no competing financial interests.
References
- 1.Thudichum JLW. A Treatise on the Chemical Constitution of Brain. Bailliere and Cox; 1884. [Google Scholar]
- 2.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature Rev. Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 5.Garofalo K, et al. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslow DK, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. This study was the first demonstration that Orm proteins are regulators of serine palmitoyltransferase, the first and rate-limiting step in sphingolipid synthesis, and provided key insights into how cells maintain sphingolipid homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffatt MF, et al. A large-scale, consortium-based genome wide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada K. Co-evolution of sphingomyelin and the ceramide transport protein CERT. Biochim. Biophys. Acta. 2014;1841:704–719. doi: 10.1016/j.bbalip.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.D’Angelo G, Rega LR, De Matteis MA. Connecting vesicular transport with lipid synthesis: FAPP2. Biochim. Biophys. Acta. 2012;1821:1089–1095. doi: 10.1016/j.bbalip.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- 11.Nakahara K, et al. The Sjogren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell. 2012;46:461–471. doi: 10.1016/j.molcel.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Kharel Y, et al. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem. J. 2011;440:345–353. doi: 10.1042/BJ20110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. The authors of this paper demonstrated that the S1P in blood is derived from haematopoietic cells, whereas lymph S1P was not, and clearly demonstrated that S1PR1 expression on lymphocytes was required for their egress. [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman K, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham TH, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza A, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuhara S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. Using global and endothelial-specific Spns2 knockout mice, these authors showed that Spns2 transports S1P out of endothelial cells and is required for B- and T-cell egress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl Acad. Sci. USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, et al. Hepatic ApoM overexpression stimulates formation of larger, ApoM/S1P-enriched plasma HDL. J. Biol. Chem. 2014;289:2801–2814. doi: 10.1074/jbc.M113.499913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurano M, et al. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 2013;229:102–109. doi: 10.1016/j.atherosclerosis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc. Natl Acad. Sci. USA. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins RW, et al. Regulation of CC ligand 5/RANTES by acid sphingomyelinase and acid ceramidase. J. Biol. Chem. 2011;286:13292–13303. doi: 10.1074/jbc.M110.163378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong Y, et al. Sphingosine kinases are not required for inflammatory responses in macrophages. J. Biol. Chem. 2013;288:32563–32573. doi: 10.1074/jbc.M113.483750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitson SM, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. This paper was the first demonstration that SphK1 is phosphorylated on Ser 225 by ERK, which is required for both activation and translocation of SphK1 to the plasma membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snider AJ, et al. A role for sphingosine kinase 1 in dextran sulfate sodium induced colitis. FASEB J. 2009;23:143–152. doi: 10.1096/fj.08-118109. This study was the first to show that S1P in colon and blood was increased in a mouse model of colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price MM, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J. Allergy Clin. Immunol. 2013;131:501–511. doi: 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature Med. 2011;17:55–63. doi: 10.1038/nm.2277. This was the first report showing that the anti-obesity and anti-inflammatory hormone adiponectin acts by stimulating ceramidase activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitano M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. erratum 54, 1704 (2006) [DOI] [PubMed] [Google Scholar]
- 31.Majumdar I, Mastrandrea LD. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine. 2012;41:442–449. doi: 10.1007/s12020-011-9589-4. [DOI] [PubMed] [Google Scholar]
- 32.Canals D, Roddy P, Hannun YA. Protein phosphatase 1α mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. J. Biol. Chem. 2012;287:10145–10155. doi: 10.1074/jbc.M111.306456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saddoughi SA, et al. Sphingosine analogue drug FTY720 targets I2PP2A/ SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox TE, et al. Ceramide recruits and activates protein kinase C ζ (PKC ζ) within structured membrane microdomains. J. Biol. Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 35.Henry B, Ziobro R, Becker KA, Kolesnick R, Gulbins E. Acid sphingomyelinase. Handb. Exp. Pharmacol. 2013;215:77–88. doi: 10.1007/978-3-7091-1368-4_4. [DOI] [PubMed] [Google Scholar]
- 36.Pettus BJ, et al. Ceramide-1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. This study reported the first indication that C1P is a direct activator of cPLA2α. [DOI] [PubMed] [Google Scholar]
- 37.Tauzin L, et al. Effects of ceramide-1-phosphate on cultured cells: dependence on dodecane in the vehicle. J. Lipid Res. 2007;48:66–76. doi: 10.1194/jlr.M600399-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Simanshu DK, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500:463–467. doi: 10.1038/nature12332. This paper identified CPTP as a specific C1P transporter that transfers C1P from the Golgi and thereby negatively regulates C1P and eicosanoid production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamour NF, et al. Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFα) via inhibition of TNFα-converting enzyme. J. Biol. Chem. 2011;286:42808–42817. doi: 10.1074/jbc.M111.310169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MJ, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. This paper reported the first identification of a S1P receptor. [DOI] [PubMed] [Google Scholar]
- 41.Hla T, Dannenberg AJ. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012;16:420–434. doi: 10.1016/j.cmet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 43.Green JA, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nature Immunol. 2011;12:672–680. doi: 10.1038/ni.2047. The authors in this paper showed that S1PR2 expression on germinal centre B cells promotes apoptosis and inhibits migration, thus implicating S1PR2 as a negative regulator of B-cell activation and maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 2014 doi: 10.1194/jlr.R046656. http://dx.doi.org/10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson MA, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. This was the first crystal structure of the lipid-binding GPCR S1PR1 that indicated that S1P enters the binding pocket by sliding in the plane of the membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. The authors of this paper demonstrated that S1P produced by SphK1 binds to and enhances the E3 ubiquitin ligase activity of TRAF2, an important component in NF-κB signalling induced by TNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harikumar KB, et al. K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nature Immunol. 2014;15:231–238. doi: 10.1038/ni.2810. This paper reported that in response to IL-1, cIAP2 and SphK1 form a complex with IRF1, leading to its activation by S1P-enhanced K63-linked polyubiquitylation, which in turn leads to the induction of IRF1-dependent chemokine genes that are important for sterile inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ihlefeld K, Claas RF, Koch A, Pfeilschifter JM, Meyer Zu Heringdorf D. Evidence for a link between histone deacetylation and Ca2+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem. J. 2012;447:457–464. doi: 10.1042/BJ20120811. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen-Tran DH, et al. Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy. Dis. Model. Mech. 2014;7:41–54. doi: 10.1242/dmm.013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrache I, Petrusca DN, Bowler RP, Kamocki K. Involvement of ceramide in cell death responses in the pulmonary circulation. Proc. Am. Thorac. Soc. 2011;8:492–496. doi: 10.1513/pats.201104-034MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. This paper reported that S1P regulates adherens junctions. [DOI] [PubMed] [Google Scholar]
- 53.Garcia JG, et al. Sphingosine-1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Natarajan V, et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1- phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2013;49:6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang QJ, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. The authors of this article provided evidence that obesity-induced endothelial dysfunction is due to elevation of ceramide, which de-represses PP2A, leading to dephosphorylation and inhibition of Akt and eNOS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Predescu S, et al. Platelet activating factor-induced ceramide micro-domains drive endothelial NOS activation and contribute to barrier dysfunction. PLoS ONE. 2013;8:e75846. doi: 10.1371/journal.pone.0075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herzog BH, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumaraswamy SB, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit. Care. 2012;16:R60. doi: 10.1186/cc11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui H, et al. Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice. J. Allergy Clin. Immunol. 2013;132:1205–1214. doi: 10.1016/j.jaci.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 61.Zhang G, et al. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013;122:443–455. doi: 10.1182/blood-2012-11-467191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teijaro JR, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl Acad. Sci. USA. 2014;111:3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol. Sci. 2011;32:16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takada K, et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J. Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faroudi M, et al. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116:5536–5547. doi: 10.1182/blood-2010-08-299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnon TI, et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–1903. doi: 10.1126/science.1208248. The authors of this paper explain how lymphocytes migrate into lymphoid tissues against the S1P gradient by GRK2-dependent downregulation of S1PR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc. Natl Acad. Sci. USA. 2010;107:20447–20452. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnon TI, Horton RM, Grigorova IL, Cyster JG. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2013;493:684–688. doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-α-induced arthritis. J. Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 74.Puneet P, et al. Retraction. Science. 2013;341:342. doi: 10.1126/science.341.6144.342-a. [DOI] [PubMed] [Google Scholar]
- 75.Zemann B, et al. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol. Lett. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Grin’kina NM, et al. Sphingosine kinase 1 deficiency exacerbates LPS-induced neuroinflammation. PLoS ONE. 2012;7:e36475. doi: 10.1371/journal.pone.0036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di A, et al. A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J. Biol. Chem. 2010;285:15848–15857. doi: 10.1074/jbc.M109.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang J, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J. Biol. Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. This paper is the first demonstration of the involvement of neutral sphingomyelinase in TNF actions. [DOI] [PubMed] [Google Scholar]
- 80.Xia P, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J. Biol. Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 81.Billich A, et al. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1β and TNF-α induced production of inflammatory mediators. Cell. Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Pettus BJ, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-α. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 83.Adada MM, et al. Sphingosine kinase 1 regulates tumor necrosis factor-mediated RANTES induction through p38 mitogen-activated protein kinase but independently of nuclear factor ΚB activation. J. Biol. Chem. 2013;288:27667–27679. doi: 10.1074/jbc.M113.489443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blackwell K, et al. Two coordinated mechanisms underlie tumor necrosis factor alpha-induced immediate and delayed IκB kinase activation. Mol. Cell. Biol. 2013;33:1901–1915. doi: 10.1128/MCB.01416-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dennis EA, et al. A mouse macrophage lipidome. J. Biol. Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sims K, et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 2010;285:38568–38579. doi: 10.1074/jbc.M110.170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali M, et al. Altering the sphingolipid acyl chain composition prevents LPS/GLN-mediated hepatic failure in mice by disrupting TNFR1 internalization. Cell Death Dis. 2013;4:e929. doi: 10.1038/cddis.2013.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 90.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Park JW, et al. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013;57:525–532. doi: 10.1002/hep.26015. [DOI] [PubMed] [Google Scholar]
- 92.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Med. 2011;17:179–188. doi: 10.1038/nm.2279. This report is the first demonstration that ceramide can activate an inflammasome, leading to caspase-1 cleavage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Youm YH, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Youm YH, et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, et al. Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res. 2014;114:792–805. doi: 10.1161/CIRCRESAHA.114.302439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holland WL, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. The authors of this paper showed that the anti-obesity hormone FGF21 acts through stimulation of adiponectin secretion that in turn decreases ceramide levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Z, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Mietla JA, et al. Characterization of eicosanoid synthesis in a genetic ablation model of ceramide kinase. J. Lipid Res. 2013;54:1834–1847. doi: 10.1194/jlr.M035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hankins JL, Fox TE, Barth BM, Unrath KA, Kester M. Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J. Biol. Chem. 2011;286:44357–44366. doi: 10.1074/jbc.M111.264010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granado MH, et al. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell. Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. This is the first demonstration that the phosphorylated form of FTY720 is an agonist for all S1P receptors, but S1PR2 and that FTY720-P induces lymphopaenia. [DOI] [PubMed] [Google Scholar]
- 103.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl Acad. Sci. USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. This study identified non-immunological central nervous system mechanisms of FTY720 efficacy in EAE that depend on the drugs ability to modulate S1PR1 expression in astrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gergely P, et al. The selective S1P receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate: translation from preclinical to clinical studies. Br. J. Pharmacol. 2012;167:1035–1047. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oo ML, et al. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsunemi S, et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 2010;136:197–204. doi: 10.1016/j.clim.2010.03.428. [DOI] [PubMed] [Google Scholar]
- 107.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. This paper provides compelling evidence that lymphocyte egress depends on the S1P gradient. [DOI] [PubMed] [Google Scholar]
- 108.Bagdanoff JT, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J. Med. Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 109.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daniel C, et al. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol. Immunol. 2007;44:3305–3316. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 111.Sanada Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS ONE. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 113.Kohno M, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawamori T, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trifilieff A, Fozard JR. Sphingosine-1-phosphate-induced airway hyper reactivity in rodents is mediated by the sphingosine-1-phosphate type 3 receptor. J. Pharmacol. Exp. Ther. 2012;342:399–406. doi: 10.1124/jpet.112.191585. [DOI] [PubMed] [Google Scholar]
- 116.Olivera A, et al. Sphingosine-1-phosphate can promote mast cell hyper reactivity through regulation of contactin-4 expression. J. Leukoc. Biol. 2013;94:1013–1024. doi: 10.1189/jlb.0313163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishiuma T, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 118.Siow DL, Wattenberg BW. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J. Biol. Chem. 2012;287:40198–40204. doi: 10.1074/jbc.C112.404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Çallışkan M, et al. Rhinovirus wheezing illness and genetic risk of childhood onset asthma. N. Engl. J. Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller M, et al. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc. Natl Acad. Sci. USA. 2012;109:16648–16653. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ha SG, et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nature Commun. 2013;4:2479. doi: 10.1038/ncomms3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masini E, et al. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J. Pharmacol. Exp. Ther. 2008;324:548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- 123.Worgall TS, et al. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci. Transl. Med. 2013;5:186ra67. doi: 10.1126/scitranslmed.3005765. This work showed that inhibition of sphingolipid biosynthesis enhances airway hyper-responsiveness. [DOI] [PubMed] [Google Scholar]