Significance
Morbilliviruses are a growing concern because of their ability to infect multiple species. The spill-over of canine distemper virus (CDV) from domestic dogs has been associated with severe declines in wild carnivores worldwide, and therefore mass dog vaccination has been suggested as a potential control strategy. Focusing on three decades of CDV exposure data in dogs and lions of the Serengeti, we show that cyclic infection dynamics in lions initially driven by dogs became more frequent and asynchronous, suggesting that the wider dog population and other wildlife species drive CDV dynamics. Hence, although widespread dog vaccination reduced the infection in dogs, transmission to lion populations still occurred, warranting further investigation into effective management options of CDV in this species-rich ecosystem.
Keywords: cross-species transmission, multihost pathogens, reservoirs, state–space models, serology
Abstract
Morbilliviruses cause many diseases of medical and veterinary importance, and although some (e.g., measles and rinderpest) have been controlled successfully, others, such as canine distemper virus (CDV), are a growing concern. A propensity for host-switching has resulted in CDV emergence in new species, including endangered wildlife, posing challenges for controlling disease in multispecies communities. CDV is typically associated with domestic dogs, but little is known about its maintenance and transmission in species-rich areas or about the potential role of domestic dog vaccination as a means of reducing disease threats to wildlife. We address these questions by analyzing a long-term serological dataset of CDV in lions and domestic dogs from Tanzania’s Serengeti ecosystem. Using a Bayesian state–space model, we show that dynamics of CDV have changed considerably over the past three decades. Initially, peaks of CDV infection in dogs preceded those in lions, suggesting that spill-over from dogs was the main driver of infection in wildlife. However, despite dog-to-lion transmission dominating cross-species transmission models, infection peaks in lions became more frequent and asynchronous from those in dogs, suggesting that other wildlife species may play a role in a potentially complex maintenance community. Widespread mass vaccination of domestic dogs reduced the probability of infection in dogs and the size of outbreaks but did not prevent transmission to or peaks of infection in lions. This study demonstrates the complexity of CDV dynamics in natural ecosystems and the value of long-term, large-scale datasets for investigating transmission patterns and evaluating disease control strategies.
The genus Morbillivirus includes highly contagious, and often fatal, RNA viruses that cause diseases of great public health, economic, and conservation concern, such as measles, rinderpest, and canine distemper. Canine distemper virus (CDV) is distributed worldwide and affects an expanding range of host species, including domestic and wild canids (1, 2), marine mammals (3), felids (2, 4, 5), procyonids and ursids (6), and nonhuman primates (7–9). The propensity of CDV for host-switching has raised concerns about both potential risks for humans (10) and extinction threats to endangered wildlife (11–13).
Although previously thought to be nonpathogenic in cats, outbreaks among large captive felids in the 1990s drew attention to CDV as a potential conservation threat to felids (2). The best-studied example of CDV infection in free-ranging felids comes from Tanzania’s Serengeti ecosystem (Fig. 1A), where a CDV epidemic in 1994 killed ∼30% of lions (Panthera leo) and affected several other carnivore species within the Serengeti National Park (SNP) (14). The close similarity of viruses recovered from wild carnivores and domestic dogs (Canis lupus familiaris) (15) indicated that a single dog variant was responsible for the die-off in lions. Prolonged viral circulation during 1992–1994 was documented in higher-density dog populations adjacent to the northwestern boundaries of the SNP with more sporadic exposure detected in lower-density dog populations to the east (exposed in 1991 and 1994 but not in 1992–1993). The high-density dog populations therefore were considered the most likely source of infection for wildlife (Fig. 1A) (16).
Fig. 1.
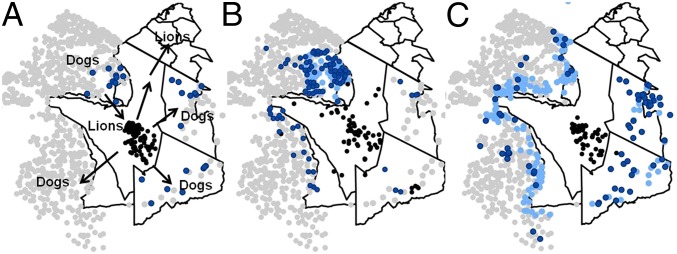
Map of the Serengeti ecosystem (Tanzania). Circles represent human settlements (gray) surrounding the Serengeti National Park, villages/households from which domestic dogs were sampled (dark blue), locations where lions were sampled (black), and villages included in domestic dog vaccination campaigns that were not sampled (pale blue). (A) Arrows indicate the direction of the spread of CDV during the 1994 epidemic as reconstructed by Cleaveland et al. (16). (B) Small-scale domestic dog vaccination campaigns conducted during 1996–2002. (C) Expanded domestic dog vaccination program implemented during 2003–2012.
Similar to other morbilliviruses [e.g., measles virus (17–19)], the acute, highly immunizing nature of CDV infection suggests that large populations of susceptible hosts are required for persistence. In smaller populations, more or less regular epidemics are typically followed by “fade-outs” during which infection disappears until reintroduced from outside (20). However, many questions remain regarding population size thresholds and determinants of CDV persistence in natural ecosystems comprising a wide range of susceptible hosts.
In the Serengeti ecosystem, earlier studies indicate that CDV was unlikely to have been maintained by lion populations or other wildlife (e.g., combined lion, hyena, and jackal populations) in the SNP before the 1994 epidemic (21, 22), further implicating higher-density domestic dogs as a more likely reservoir of infection (16). However, observations from other ecosystems do not support this hypothesis. In northern Kenya, for example, domestic dog populations adjacent to wildlife protected areas show patterns of exposure consistent with reoccurring outbreaks rather than persistent infection (23), suggesting that the dog population is insufficiently large to maintain CDV and that infection needs to be reintroduced from outside sources (e.g., other domestic dog or wildlife communities). In other large, protected areas such as the Yellowstone National Park, the periodic nature of CDV occurrence in wild carnivore communities and the small size of the dog population around the park suggest disease persistence in the wild populations themselves (24).
Knowledge of the mechanisms of long-term CDV maintenance is essential to optimize disease management (25). Mass vaccination of domestic dogs has been proposed as a strategy for protecting endangered wildlife in many areas (26, 27), but concerns arise over its cost-effectiveness as a conservation tool within a potentially complex system, especially when the contribution of dogs to disease maintenance is uncertain (28). The implementation of mass dog vaccination programs in the Serengeti ecosystem since 1996, although driven largely by the need to control rabies (29), provides an opportunity to evaluate the impact of dog vaccination on CDV infection in both dog and wildlife populations.
In this study we analyze the most comprehensive available multihost dataset on CDV in Africa, including domestic dog and lion serology data from the Serengeti ecosystem spanning almost three decades as well as data from mass dog vaccination interventions. These data are analyzed using a Bayesian state–space modeling approach to examine long-term patterns of infection in a large, multihost ecosystem. We first determine the role of domestic dogs and lions in maintaining CDV by assessing the within- and between-species dynamics and subsequently investigate the impact of small- (Fig. 1B) and large-scale (Fig. 1C) vaccination programs on infection dynamics.
Results
We used a Bayesian state–space model to analyze CDV serology records of lions and domestic dogs (Fig. 2) to estimate and characterize the annual probability of CDV infection for individuals of each species. The estimated annual pattern of CDV infection was best explained by a model comprising, for each species, a linear trend on time, a second-order autoregressive (AR) component that phenomenologically captures the within-species disease dynamics, a cross-species transmission parameter (lions-to-dogs and dogs-to-lions), and, for dogs, village- and region-level vaccination with a lag of 1 and 2 years.
Fig. 2.
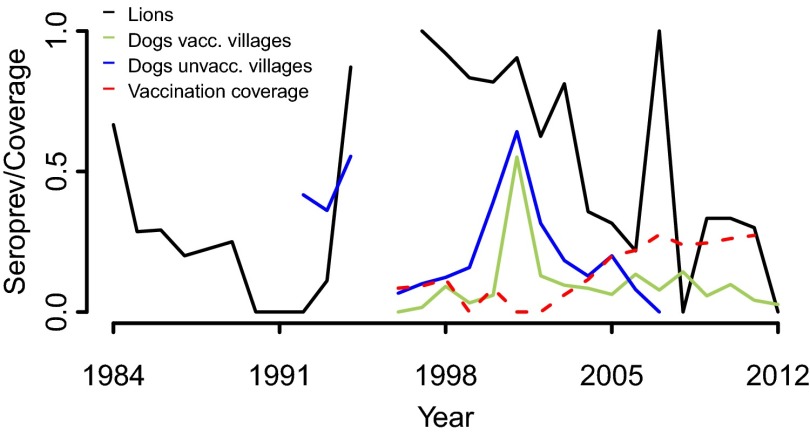
Annual CDV seroprevalence in Serengeti lions (black) and in nonvaccinated dogs from vaccinated (green) and unvaccinated (blue) villages and regional vaccination coverage (red).
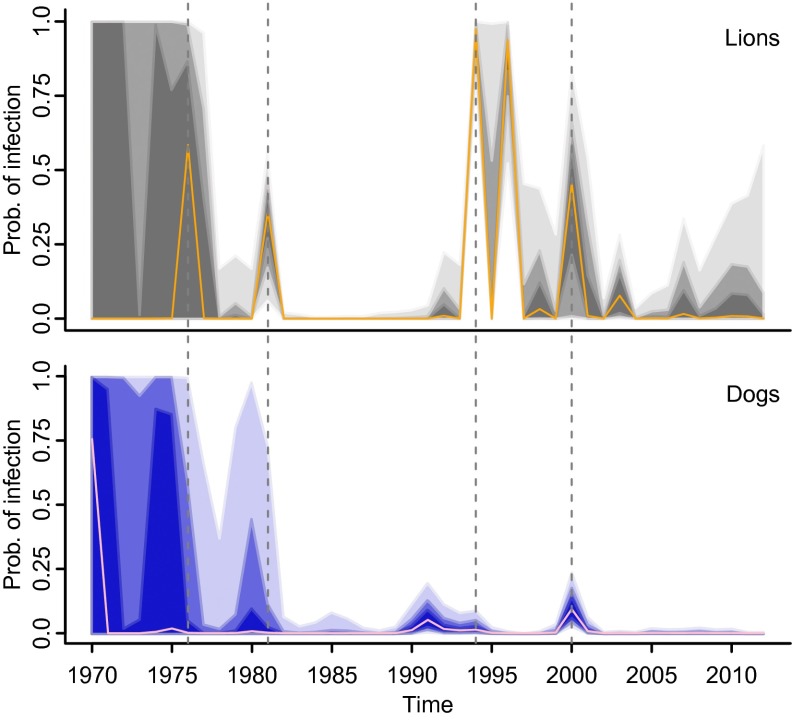
Our results show distinct changes in the pattern of the estimated annual probability of infection of dogs and lions (Fig. 3), suggesting that the mechanisms of CDV maintenance in this system have changed over the last three decades. Although CDV dynamics from 1970 to ∼1977 were uncertain [see large credible intervals (CIs) in Fig. 3], because of the lack of data during this period, there were at least two distinct episodes of exposure in lions and dogs before the mid-1990s (∼1981 and ∼1993) and, as previously observed by Packer, et al. (30), a possible third episode in ∼1976, separated by periods during which infection apparently was absent. In each of these episodes, infection peaks in dogs preceded infection peaks in lions, suggesting that during this period CDV dynamics were driven by domestic dogs and supporting the hypothesis that spill-over from domestic dogs caused the 1994 outbreak in the lion population (16). However, these dynamics changed after 1994, with a less consistent relationship between the timing of infection peaks in dogs and lions suggesting that the targeted dog population is not the only source of CDV in wildlife. The concurrent timing of exposure in dogs and lions around 2000 (Fig. 3) corresponded to increased levels of clinical infection in the dog populations and in the Ngorongoro lion population (east of SNP) (31). Lower levels of infection in lions in 1998 and 2007 (Fig. 3) also coincided with localized viral circulation in domestic dogs and African wild dog (Lycaon pictus) populations (32), although seropositivity in domestic dogs was too low to detect an increase in the annual probability of infection at these times (for low probabilities, see SI Appendix, Fig. S4).
Fig. 3.
State–space model estimates of the annual mode probability of CDV infection in lions (orange line, Upper) and dogs (pink line, Lower). Associated 50%, 75%, and 95% CIs are indicated by dark, medium, and light shading, respectively.
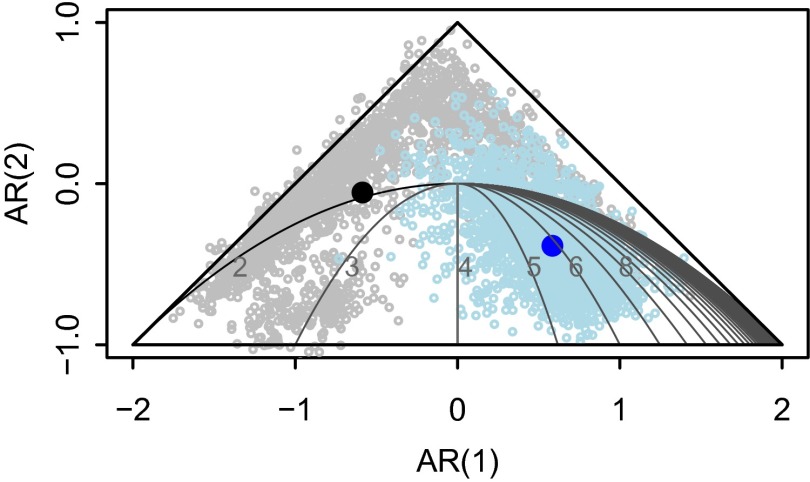
Based on the AR parameter plane (Fig. 4) (33) obtained from the estimated AR coefficients, CDV dynamics in both species exhibit damped fluctuations. However, although CDV dynamics in domestic dogs are cyclic, with a period of ∼6 years between peaks of infection (Fig. 4), the dynamics in lions are less distinctly cyclic (i.e., AR coefficients fall adjacent to the parameter plane parabola) (Fig. 4), and hence, if present, cyclicity in lions exhibits a higher frequency with a mean of 2 years between peaks of infection. This difference suggests distinct maintenance and/or transmission mechanisms in these species. The model results also showed a consistently lower mean annual probability of infection in dogs than in lions (Fig. 3; see difference between dog and lion probability of infection in SI Appendix, Fig. S5) and narrower CIs, because sampling was more systematic and sample sizes were larger in dogs than in lions. In addition, lions are longer lived and have a higher chance of exposure throughout their lifetime.
Fig. 4.
AR parameter plane. Closed dark dots correspond to the posterior mean of the first-order AR coefficient [x axis, AR (1)] of the best model (i.e., ω2/β2) against the posterior mean of the second-order AR coefficient [y axis, AR (2)] (i.e., ω3/β3) for dogs (blue) and lions (black), respectively. Each open light dot corresponds to a draw of the AR posterior distributions. Parameters outside the triangle indicate unstable dynamics that become extinct. Inside the triangle, the dynamics are stable or display damped oscillations. Within the parabola, the dynamics are cyclic with the period increasing from left to right as represented by the contour lines.
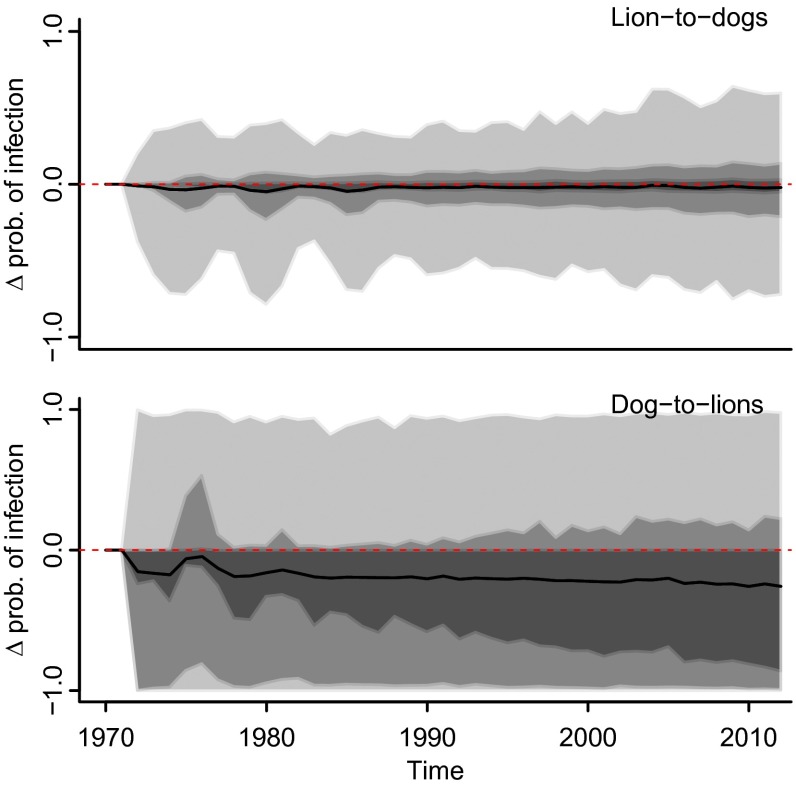
To investigate the impact of cross-species transmission on CDV dynamics, we compared the forecast of the annual probabilities of infection with and without the cross-species transmission parameter (Fig. 5). The similarity between the predicted probability of infection in dogs with and without lion-to-dog transmission, i.e., the mean difference between the two, is close to zero, and CIs span an equal range above and below zero (Fig. 5, Upper), indicating that transmission from lions to dogs is negligible. This finding is consistent with the estimated lion-to-dog transmission parameter value [ω4 = 0.032 (0.00–0.20)] (SI Appendix, Table S5). The lion prediction model was imprecise (see the CIs in Fig. 5, Lower that range from −1 to 1), probably because of small sample sizes, and therefore was uninformative. However, the effect size of the parameter governing dog-to-lion transmission [β4 = 0.283 (0.08–2.48)] was 10 times larger than that of lion-to-dog transmission, indicating strongly asymmetric cross-species transmission.
Fig. 5.
Sensitivity analysis results for the cross-species transmission parameter showing the difference between the probability of dog infection predicted with and without lion-to-dog transmission (Upper) and between the probability of lion infection predicted with and without dog-to-lion transmission (Lower). Shaded areas around the mean (black line) correspond to 50% (dark gray), 75% (medium gray), and 95% (light gray) CIs.
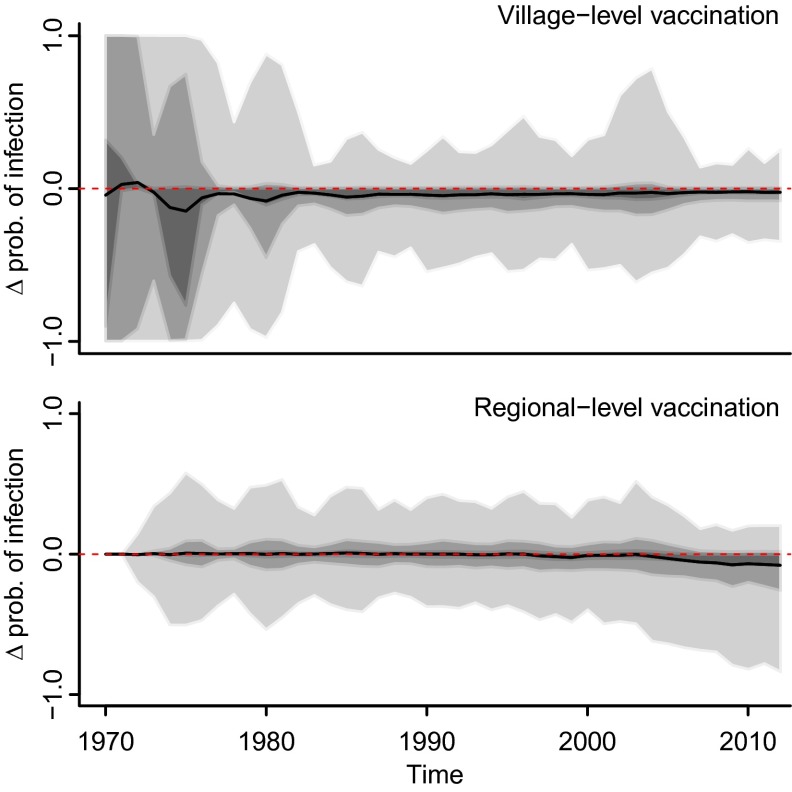
To investigate the role of dog vaccination on CDV dynamics in dogs, we compared the forecasts of the annual probability of infection with and without vaccination at the village (i.e., binary indicator of dog CDV vaccination in the village) and regional (i.e., annual dog vaccination coverage across all villages sampled within the Serengeti ecosystem) levels. Initially (1996–2002), dog vaccination programs targeted only dog populations to the northwest of SNP (Fig. 1B) and covered a limited and patchy area, especially during 2000–2002 (Fig. 2, red line). From 2003, an extended vaccination program attained a more consistent spatial coverage by encompassing all villages within a 10-km zone adjacent to the western boundaries of SNP and all villages to the east (Figs. 1C and 2). Our results (Fig. 6, Upper) indicate that CDV dynamics in domestic dogs are not obviously influenced by the local vaccination status of villages; the mean difference between the predicted annual probability of infection with and without village-level vaccination was narrowly (but consistently) below zero, with the lower and upper CIs only slightly asymmetric around zero (Fig. 6, Upper). Together with the apparent natural fade-out of CDV in dogs from unvaccinated villages during 1996–2000 and the limited exposure in younger animals up until 2000 (SI Appendix, Fig. S9), when reintroductions of infection were observed (Fig. 3), our results also point to a negligible effect of regional-level vaccination when efforts are patchy and limited (1996–2002) (Fig. 6, Lower). However, continuous and more extensive vaccination coverage (∼30%), as implemented from 2003 onwards (Fig. 2), has a clearly identifiable impact on CDV infection in dogs, as demonstrated by the ∼5% decrease in the predicted mean difference of the probability of infection with and without regional-level vaccination from 2003 onwards (Fig. 6, Lower; for raw predictions see SI Appendix, Fig. S8). In addition, ∼70% of the posterior draws of the difference between the predicted probability of infection with and without regional-level vaccination from 2003 onward were negative (Fig. 6, Lower), suggesting that CDV outbreaks in domestic dogs could be much larger in the absence of continuous and extensive vaccination. The change in the final upper CIs of SI Appendix, Fig. S8, from a maximum of 0.4 with vaccination to 0.9 without vaccination, show that the outbreaks could be up to 50% larger.
Fig. 6.
Results of sensitivity analysis for the vaccination parameters showing the difference between the probability of dog infection predicted with and without village-level vaccination (Upper) and with and without regional-level vaccination (Lower). Shaded areas around the mean (black line) correspond to 50% (dark gray), 75% (medium gray), and 95% (light gray) CIs.
Because of the uncertainty in the lion prediction model (Fig. 5, Lower), it was not possible to determine directly whether dog-to-lion transmission was affected by dog vaccination, but the intensity of CDV outbreaks in lions apparently was lower after the establishment of the mass vaccination program (2003 onwards) (Fig. 3), suggesting a lower force of infection from dogs to lions. However any reduction was insufficient to prevent the disease from circulating in lions altogether and may have been concealed by smaller sample sizes.
Discussion
This study presents an unprecedented dataset and epidemiological analyses of morbillivirus transmission dynamics at the wildlife–domestic animal interface. The findings indicate that (i) over almost four decades, cross-species transmission of CDV in the Serengeti ecosystem has been dominated by dog-to-lion transmission, although some lion-to-dog transmission is also likely to have occurred; (ii) CDV dynamics are cyclic in both dogs and lions, although lion dynamics exhibit a much higher periodicity of cycles than dogs, suggesting distinct maintenance and/or transmission mechanisms; (iii) the relationship between the timing of infection in dogs and lions has changed with time, and a lack of synchrony in infection peaks in dogs and lions may be explained by the different periodicities of infection dynamics; (iv) small-scale dog vaccination (1996–2002) had little or no effect on regional CDV dynamics in dogs, but larger-scale campaigns (2003–2012) had a significant impact, potentially halving the size of outbreaks in dogs; (v) neither small- nor larger-scale dog vaccination campaigns prevented transmission of CDV infection to lions; and (vi) domestic dog populations immediately surrounding the SNP are not the sole driver of CDV infection in lions, and CDV persistence is likely to involve a larger multihost community.
Morbilliviruses are a fascinating group of pathogens that include viruses that have been eradicated (e.g., rinderpest virus, RPV), those that are well understood and controllable through mass vaccination (e.g., measles virus), and those that are emerging in new host populations and in new areas, with changing patterns of pathogenicity and transmission (e.g., CDV and marine mammal morbilliviruses) (for a review see ref. 10). The feasibility of eliminating and controlling morbilliviruses through mass vaccination depends largely on the nature of the reservoir system. Both RPV and measles viruses are maintained by single-host populations (cattle and humans, respectively). In contrast, this study demonstrates that the potential complexity of CDV maintenance patterns in multihost ecosystems, such as the Serengeti, poses substantial challenges for control or elimination.
Earlier studies pointed to domestic dogs as a potential reservoir of CDV in the Serengeti ecosystem (16) and as a target for interventions. Our analysis demonstrates that, at least in the last two decades, dogs from the Serengeti ecosystem were unlikely to be the sole source of infection for wildlife. Previous studies showed that in the Serengeti ecosystem the lion population is too small to maintain CDV on its own (21, 22). Together with these earlier reports, our finding that CDV can circulate in lions even when levels of infection are extremely low and asynchronous in domestic dogs supports the hypothesis that CDV infection in the Serengeti ecosystem is likely to persist across large regional scales, involving the wider domestic dog population beyond the Serengeti ecosystem and other wildlife species. Although our study focuses only on lions, the broader wild carnivore community, comprising more than 28 species, is likely to play an important role in transmission of CDV in the ecosystem (34). Wild carnivores, such as hyena, jackal, and mongoose species (35), which are abundant in villages adjacent to SNP, likely comprise numerous “liaison” hosts linking domestic dogs with lions. However, questions remain about the relative role of wild and domestic carnivores in CDV persistence. For example, the long gap in exposure to CDV in dogs and lions during the 1980s suggests that CDV disappeared from the ecosystem for a prolonged period, and therefore it is unlikely that wild carnivores acted as maintenance communities during this time. Since then the situation is less clear, with only a short period (∼2005) when CDV disappeared from lions. Similarly, there is no evidence for continuous circulation of CDV in the sampled dog populations living in proximity to the protected areas, suggesting that these populations are not capable of independent maintenance. Combined, these observations lead to the hypothesis that the larger, mostly unvaccinated, dog populations outside the study area may contribute to a maintenance community that also comprises other wild carnivores.
The reasons for the shifts in CDV dynamics following the 1994 epidemic are unclear. A higher frequency of infection peaks in lions, despite low levels of infection in domestic dogs, could have been the result of higher, more consistent levels of infection in other wild carnivore hosts. However, carnivore transect counts in the SNP provide no evidence for a change in carnivore assemblages or host density that might indicate more sustained circulation and maintenance of CDV in wildlife (35). The nonstationary patterns of CDV infection resemble the dynamics of other morbilliviruses, e.g., measles, before and after mass immunization efforts (19, 36, 37). Reductions in pools of susceptibles as a result of vaccination were important determinants in the temporal transitions in measles dynamics (from regular to irregular cycles) in England and Wales (19, 38, 39). Therefore mass vaccination targeting domestic dog populations also might explain the changing CDV dynamics in the Serengeti. However, our model indicates that small-scale vaccination campaigns conducted during 1997–2002 had little or no impact on the probability of CDV infection, and an increased frequency of peaks in lions was already observable before the implementation of large-scale vaccination campaigns in 2003. Combined, these results suggest that changing CDV dynamics in lions are unlikely to be related to mass dog vaccination, i.e., there is no evident causal relationship between shifts in lion CDV dynamics and dog vaccination. Natural CDV cycles or increasing human and associated dog populations in villages across northern Tanzania provide alternative possible explanations for the shifting patterns of infection.
Previous studies in the Serengeti ecosystem have highlighted domestic dogs as the potential reservoir of both CDV and rabies, another multihost viral pathogen of carnivores (40). Although studies indicate that the Serengeti dog population is the sole maintenance population of rabies (41), the same does not appear to be true for CDV. Each dog vaccinated in this study area receives both rabies and CDV vaccine, and although this dual vaccination has been sufficient to eliminate rabies from lower-density dog populations and wildlife to the east of SNP, with long periods of absence from wildlife in SNP (42), CDV continues to circulate in wildlife and, although to a lesser extent, in domestic dogs in these areas. This persistence is likely caused by a higher basic reproductive number (R0) for CDV compared with rabies (43), as suggested by R0 estimates for other morbilliviruses [e.g., phocine distemper virus (44)].
Larger-scale and continuous vaccination programs may reduce the mean annual probability of infection in dogs by ∼5% and the size of potential outbreaks, highlighting the importance of the expanded vaccination program (covering 30,000–50,000 dogs each year in >200 villages) in maintaining the current low levels of CDV circulation in domestic dog populations. However, even this level of dog vaccination does not seem to prevent transmission to lions, because infection peaks continue to occur, although seemingly with lower amplitudes than during the prevaccination era. The complexities and shifting patterns of CDV dynamics in the Serengeti ecosystem raise many questions as to the most appropriate and cost-effective approaches for the management of CDV in natural ecosystems. Although concerns about the impact of CDV in lions were raised because of high mortality in the 1994 outbreak, there currently is no evidence of clinical impacts of CDV infection in lions, except when outbreaks are synchronized with high levels of Babesia spp. (31). However, concerns remain about the vulnerability of critical populations, such as African wild dogs (45). Our results suggest that, as a conservation measure to protect wildlife, mass domestic dog vaccination efforts need to be continuous and widespread, posing logistic and financial challenges, and, even then, are unlikely to result in the elimination of infection in wildlife-protected areas.
Despite ongoing debates about the risks of vaccinating threatened wildlife (e.g., ref. 26), substantial progress has been made in developing efficient and safe vaccines for use in a range of carnivores (46, 47), which may be considered as an alternative disease management strategy. Mathematical models suggest that vaccinating a core (i.e., 30–40%) of individuals against rabies in endangered African wild dog and Ethiopian wolf (Canis simensis) populations would be sufficient to ensure the persistence of small populations (48, 49). A policy of core vaccination strategies against CDV in these species could also be a feasible and more cost-effective strategy than mass dog vaccination for protecting endangered populations against extinction risks.
Serological approaches are key to assessing exposure of populations to CDV (50). However, our study raises a number of issues with respect to sampling strategies for CDV surveillance in domestic dog populations. The patchiness and low rates of infection indicate that a larger number of villages may need to be sampled to be able to detect the disappearance or spread of CDV in any given area. However, given the limited resources available for serological testing, increased sampling of villages is typically offset by smaller sample sizes within villages. This study demonstrates the value of combining long-term serological data with advanced analytical tools to maximize the utility of these serological data and to explain complex patterns of infection.
Cross-reactivity is an issue common to all serology studies (50). For example, sera from cattle infected with morbilliviruses such as RPV have been shown to neutralize CDV (51), and serological tests cannot easily distinguish antibodies against CDV from antibodies against RPV. Lions sampled before RPV eradication could have been exposed to RPV (e.g., through consumption of infected carcasses) and therefore could have detectable CDV titers in the absence of CDV infection. However, the clear episodic pattern of CDV infection during those years, together with the low CDV exposure in lions in the years following the last known RPV outbreak in the region (1982–1983) (52), and the inclusion of a probability of misclassification of disease status in the modeling framework, limit the potential role of misclassification of CDV infection resulting from cross-immunity with RPV. Furthermore, a refit of the model excluding data from before 1983 resulted in similar patterns of CDV infection post-1983.
The integration of state-of-the-art analytical methods with data from large-scale monitoring and intervention studies provided a unique opportunity to explore long-term CDV dynamics and the impacts of interventions at the domestic–wildlife interface in a species-rich ecosystem. Our findings have important implications for future research on CDV and other challenging multihost systems and provide directions for the management of endangered wildlife, especially those at the domestic–wildlife interface.
Materials and Methods
Lion Data.
Lion data included CDV serology data from lions sampled from 1984–2012 (n = 535) as part of SNP management or research operations, and years of sampling and birth. Further details are provided in SI Appendix.
Domestic Dog Data.
Domestic dog data included CDV serology data, village-level vaccination efforts (number of dogs vaccinated), and years of sampling and birth of each dog. Dogs were sampled from 1992–2012 (n = 6,866) during central-point and house-to-house vaccination campaigns (29) and, in unvaccinated areas, during randomized household surveys. Further details are provided in SI Appendix.
Serological Assays.
CDV serology was carried out using neutralization assays at Intervet (United Kingdom), Animal Health Diagnostic Center (Cornell University, Ithaca, NY), and University of Glasgow (United Kingdom). We used a cutoff titer value equivalent to a 1:16 dilution to define prior exposure, as in other studies of CDV exposure in wild carnivore species (23, 24). Fig. 2 shows annual seroprevalence in lions and dogs. Further details are provided in SI Appendix.
Intervention Studies.
Domestic dog vaccination programs against rabies, CDV, and canine parvovirus have been carried out simultaneously since 1996. Initially (1996–2002) small-scale campaigns were conducted in only one district to the northwest of SNP (Fig. 1B). From 2003 onwards, vaccination campaigns have been expanded to include all villages to the east of SNP and within a 10-km zone bordering the western boundaries of the park (Fig. 1C). Regional vaccination coverage, estimated as the ratio between the total number of vaccinated dogs and the dog population size from all sampled villages independently of vaccination history (light gray villages in Fig. 1), as well as seroprevalence over time in unvaccinated dogs from vaccinated and nonvaccinated villages, are shown in Fig. 2.
Bayesian State–Space Model.
A Bayesian state–space model was developed to estimate the (unobserved) annual probability of infection of dogs and lions and to evaluate the impact of cross-species transmission and of the vaccination program on this probability (SI Appendix). The model comprises two coupled parts, a biological and an observation process. The biological process captures the infection dynamics through a linear predictor comprising autocovariates (i.e., first- and second-order AR components capable of reconstructing endemic disease outbreaks), cross-species transmission (i.e., operating with a 1-year lag on the other species, lion-to-dog and dog-to-lion transmission), the external force of infection (accounting for species other than dogs and lions and implemented as a linear trend), and an additional region-level vaccination term exclusive to dogs (i.e., covariate of annual vaccination coverage of the previous year and 2 years before estimated across all sampled villages). The model’s observation process confronts the population-level model of the biological process with individual-level data (i.e., CDV serology data), simultaneously capturing known or suspected biases and imprecisions in the data-collection process.
Our model selection procedure considers several criteria: (i) biological plausibility; (ii) numerical robustness; (iii) goodness-of-fit; (iv) parsimony; and (v) robustness of parameter posteriors. Briefly, the model chosen (described above) is one that is biologically plausible and addresses our scientific questions but also converges well, generates validated fits, and is not identified as obviously overparameterized. Further details are given in SI Appendix.
Sensitivity Models.
To investigate the impact of cross-species transmission and vaccination on the annual probability of infection, we developed prediction models (SI Appendix) based on the best model as decided via model selection. Specifically, to investigate the quantitative effect of removing cross-species transmission, we compared the estimated annual probability of infection for dogs from the best model with that from a model with the lion-to-dog and dog-to-lion transmission parameters set to zero. To investigate the quantitative impact of vaccination, we compared the estimated annual probability of infection for dogs from the best model with that from a model with the village-level and regional-level vaccination parameters set to zero.
Supplementary Material
Acknowledgments
We thank the Tanzania Ministry of Livestock and Fisheries Development (MoLFD), Tanzania National Parks (TANAPA), Tanzania Wildlife Research Institute (TAWIRI), Ngorongoro Conservation Area Authority, and Tanzania Commission for Science and Technology for permissions and TANAPA Veterinary Unit, TAWIRI-Messerli Foundation Wildlife Veterinary Programme, Viral Transmission Dynamics Project, Serengeti Lion Project, Frankfurt Zoological Society, Serengeti Health Initiative, and MoLFD District Veterinary Offices in Mara, Mwanza, Shinyanga, and Arusha Regions for assistance with field activities. We thank William Baxendale, Stuart Chalmers, Ian Tarpey, and Claire Chillingworth (Intervet, United Kingdom) for assistance with serological analyses and Matthew Denwood for helpful discussions. We especially thank an anonymous reviewer for invaluable comments, which have improved the paper greatly. This work initially was supported by the joint NIH/National Science Foundation (NSF) Ecology of Infectious Diseases Program under NSF Grant DEB0225453. Additional support was received from the Lincoln Park Zoo, Washington State University, Tusk Trust, World Society for the Protection of Animals, Paradise Wildlife Park, Department for International Development (DFID, UK), MSD Animal Health, the Wellcome Trust, the Research and Policy for Infectious Disease Dynamics Program of the Science and Technology Directorate, the Department of Homeland Security, and the Fogarty International Center at the NIH. M.V. is funded by a Newton International Fellowship from the Royal Society. K.H. is funded by the Wellcome Trust (Fellowship 095787/Z/11/Z).
Footnotes
Conflict of interest statement: Since 2003, the project has received donations of vaccines for the mass dog vaccination campaigns from MSD Animal Health (formerly Intervet and Intervet Schering-Plough).
This article is a PNAS Direct Submission.
Data deposition: Our manuscript uses three decades of serology data from lions and dogs collected by multiple projects and governmental institutions. Some of the data are considered sensitive, and we do not have full approval to make them publicly available. However, we can share anonymized data upon request by individual readers. For data requests please email one of the corresponding authors.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411623112/-/DCSupplemental.
References
- 1.Alexander KA, Appel MJG. African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. J Wildl Dis. 1994;30(4):481–485. doi: 10.7589/0090-3558-30.4.481. [DOI] [PubMed] [Google Scholar]
- 2.Appel MJ, et al. Canine distemper epizootic in lions, tigers, and leopards in North America. J Vet Diagn Invest. 1994;6(3):277–288. doi: 10.1177/104063879400600301. [DOI] [PubMed] [Google Scholar]
- 3.Osterhaus ADME, et al. Morbillivirus infections of aquatic mammals: Newly identified members of the genus. Vet Microbiol. 1995;44(2-4):219–227. doi: 10.1016/0378-1135(95)00015-3. [DOI] [PubMed] [Google Scholar]
- 4.Daoust P-Y, McBurney SR, Godson DL, van de Bildt MWG, Osterhaus ADME. Canine distemper virus-associated encephalitis in free-living lynx (Lynx canadensis) and bobcats (Lynx rufus) of eastern Canada. J Wildl Dis. 2009;45(3):611–624. doi: 10.7589/0090-3558-45.3.611. [DOI] [PubMed] [Google Scholar]
- 5.Seimon TA, et al. Canine distemper virus: An emerging disease in wild endangered Amur tigers (Panthera tigris altaica) MBio. 2013;4(4):e00410–13. doi: 10.1128/mBio.00410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deem SL, Spelman LH, Yates RA, Montali RJ. Canine distemper in terrestrial carnivores: A review. J Zoo Wildl Med. 2000;31(4):441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Morikawa S, et al. Outbreak of deadly canine distemper virus infection among imported cynomolgus (crab-eating) monkeys. Infect Ag Surveil Rep. 2008;29:315. [Google Scholar]
- 8.Qiu W, et al. Canine distemper outbreak in rhesus monkeys, China. Emerg Infect Dis. 2011;17(8):1541–1543. doi: 10.3201/eid1708.101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, et al. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet Microbiol. 2010;141(3-4):374–378. doi: 10.1016/j.vetmic.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 10.de Swart RL, Duprex WP, Osterhaus ADME. Rinderpest eradication: Lessons for measles eradication? Curr Opin Virol. 2012;2(3):330–334. doi: 10.1016/j.coviro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343(6167):1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 12.Woodroffe R. Managing disease threats to wild mammals. Anim Conserv. 1999;2(3):185–193. [Google Scholar]
- 13.Woodroffe R, Ginsberg JR. Conserving the African wild dog Lycaon pictus. I. Diagnosing and treating causes of decline. Oryx. 1999;33(2):132–142. [Google Scholar]
- 14.Roelke-Parker ME, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379(6564):441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter MA, et al. Genetic characterization of canine distemper virus in Serengeti carnivores. Vet Immunol Immunopathol. 1998;65(2-4):259–266. doi: 10.1016/s0165-2427(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 16.Cleaveland S, et al. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Vet Microbiol. 2000;72(3-4):217–227. doi: 10.1016/s0378-1135(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett MS. Measles periodicity and community size. J Roy Stat Soc A. 1957;120(1):48–70. [Google Scholar]
- 18.Bartlett MS. The critical community size for measles in the United States. J Roy Stat Soc A. 1960;123(1):37–44. [Google Scholar]
- 19.Bolker BM, Grenfell BT. Impact of vaccination on the spatial correlation and persistence of measles dynamics. Proc Natl Acad Sci USA. 1996;93(22):12648–12653. doi: 10.1073/pnas.93.22.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd-Smith JO, et al. Should we expect population thresholds for wildlife disease? Trends Ecol Evol. 2005;20(9):511–519. doi: 10.1016/j.tree.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Craft ME, Hawthorne PL, Packer C, Dobson AP. Dynamics of a multihost pathogen in a carnivore community. J Anim Ecol. 2008;77(6):1257–1264. doi: 10.1111/j.1365-2656.2008.01410.x. [DOI] [PubMed] [Google Scholar]
- 22.Craft ME, Volz E, Packer C, Meyers LA. Distinguishing epidemic waves from disease spillover in a wildlife population. Proc Roy Soc B. 2009;276(1663):1777–1785. doi: 10.1098/rspb.2008.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prager KC, et al. Rabies virus and canine distemper virus in wild and domestic carnivores in Northern Kenya: Are domestic dogs the reservoir? EcoHealth. 2012;9(4):483–498. doi: 10.1007/s10393-013-0815-9. [DOI] [PubMed] [Google Scholar]
- 24.Almberg ES, Mech LD, Smith DW, Sheldon JW, Crabtree RL. A serological survey of infectious disease in Yellowstone National Park’s canid community. PLoS ONE. 2009;4(9):e7042. doi: 10.1371/journal.pone.0007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg Infect Dis. 2002;8(12):1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleaveland S, Kaare M, Knobel D, Laurenson MK. Canine vaccination—providing broader benefits for disease control. Vet Microbiol. 2006;117(1):43–50. doi: 10.1016/j.vetmic.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Quigley KS, et al. Morbillivirus infection in a wild Siberian tiger in the Russian Far East. J Wildl Dis. 2010;46(4):1252–1256. doi: 10.7589/0090-3558-46.4.1252. [DOI] [PubMed] [Google Scholar]
- 28.Belsare AV, Gompper ME. Assessing demographic and epidemiologic parameters of rural dog populations in India during mass vaccination campaigns. Prev Vet Med. 2013;111(1-2):139–146. doi: 10.1016/j.prevetmed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Kaare M, et al. Rabies control in rural Africa: Evaluating strategies for effective domestic dog vaccination. Vaccine. 2009;27(1):152–160. doi: 10.1016/j.vaccine.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer C, et al. Viruses of the Serengeti: Patterns of infection and mortality in African lions. J Anim Ecol. 1999;68(6):1161–1178. [Google Scholar]
- 31.Munson L, et al. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS ONE. 2008;3(6):e2545. doi: 10.1371/journal.pone.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goller KV, et al. Fatal canine distemper infection in a pack of African wild dogs in the Serengeti ecosystem, Tanzania. Vet Microbiol. 2010;146(3-4):245–252. doi: 10.1016/j.vetmic.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Royama T. Analytical Population Dynamics. Chapman & Hall; London: 1992. p. 371. [Google Scholar]
- 34.Durant SM, et al. Does size matter? An investigation of habitat use across a carnivore assemblage in the Serengeti, Tanzania. J Anim Ecol. 2010;79(5):1012–1022. doi: 10.1111/j.1365-2656.2010.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durant SM, et al. Long-term trends in carnivore abundance using distance sampling in Serengeti National Park, Tanzania. J Appl Ecol. 2011;48(6):1490–1500. [Google Scholar]
- 36.Bjørnstad ON, Finkenstädt BF, Grenfell BT. Dynamics of measles epidemics: Estimating scaling of transmission rates using a time series SIR model. Ecol Monogr. 2002;72(2):169–184. [Google Scholar]
- 37.Grenfell B, Harwood J. (Meta)population dynamics of infectious diseases. Trends Ecol Evol. 1997;12(10):395–399. doi: 10.1016/s0169-5347(97)01174-9. [DOI] [PubMed] [Google Scholar]
- 38.Keeling MJ, Grenfell BT. Disease extinction and community size: Modeling the persistence of measles. Science. 1997;275(5296):65–67. doi: 10.1126/science.275.5296.65. [DOI] [PubMed] [Google Scholar]
- 39.Keeling MJ, Grenfell BT. Understanding the persistence of measles: Reconciling theory, simulation and observation. Proc Biol Sci. 2002;269(1489):335–343. doi: 10.1098/rspb.2001.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleaveland S, Dye C. Maintenance of a microparasite infecting several host species: Rabies in the Serengeti. Parasitology. 1995;111(Suppl):S33–S47. doi: 10.1017/s0031182000075806. [DOI] [PubMed] [Google Scholar]
- 41.Lembo T, et al. Exploring reservoir dynamics: A case study of rabies in the Serengeti ecosystem. J Appl Ecol. 2008;45(4):1246–1257. doi: 10.1111/j.1365-2664.2008.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lembo T, et al. The feasibility of canine rabies elimination in Africa: Dispelling doubts with data. PLoS Negl Trop Dis. 2010;4(2):e626. doi: 10.1371/journal.pntd.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampson K, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7(3):e53. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swinton J, Harwood J, Grenfell B, Gilligan CA. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. J Anim Ecol. 1998;67(1):54–68. [Google Scholar]
- 45.van de Bildt MW, et al. Distemper outbreak and its effect on African wild dog conservation. Emerg Infect Dis. 2002;8(2):211–213. doi: 10.3201/eid0802.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardo MC, Bauman JE, Mackowiak M. Protection of dogs against canine distemper by vaccination with a canarypox virus recombinant expressing canine distemper virus fusion and hemagglutinin glycoproteins. Am J Vet Res. 1997;58(8):833–836. [PubMed] [Google Scholar]
- 47.Wimsatt J, Biggins D, Innes K, Taylor B, Garell D. Evaluation of oral and subcutaneous delivery of an experimental canarypox recombinant canine distemper vaccine in the Siberian polecat (Mustela eversmanni) J Zoo Wildl Med. 2003;34(1):25–35. doi: 10.1638/1042-7260(2003)34[0025:EOOASD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Haydon DT, et al. Low-coverage vaccination strategies for the conservation of endangered species. Nature. 2006;443(7112):692–695. doi: 10.1038/nature05177. [DOI] [PubMed] [Google Scholar]
- 49.Vial F, Cleaveland S, Rasmussen G, Haydon DT. Development of vaccination strategies for the management of rabies in African wild dogs. Biol Conserv. 2006;131(2):180–192. [Google Scholar]
- 50.Gilbert AT, et al. Deciphering serology to understand the ecology of infectious diseases in wildlife. EcoHealth. 2013;10(3):298–313. doi: 10.1007/s10393-013-0856-0. [DOI] [PubMed] [Google Scholar]
- 51.Örvell C, Norrby E. Further studies on the immunologic relationships among measles, distemper, and rinderpest viruses. J Immunol. 1974;113(6):1850–1858. [PubMed] [Google Scholar]
- 52.Dobson AP. The ecology and epidemiology of rinderpest virus in Serengeti and Ngorongoro Conservation Area. In: Sinclair ARE, Arcese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. Univ of Chicago Press; Chicago: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.