Significance
Notch signaling pathway plays crucial roles in cell-fate determination during embryonic development and cancer progression. According to the current paradigm, the Notch–Delta signaling leads to complementary cell-fate selection between two neighboring cells where one acts as Sender or Receiver. However, this picture is not complete because an additional ligand, Jagged, is involved in the Notch signaling. We devise a specific theoretical framework to decipher the functional role of Jagged. We find that the asymmetry between the modulations of Delta and Jagged leads to the existence of the previously unexplored possibility of a Sender–Receiver phenotype enabling two interacting cells to share a similar fate. This realization can provide important clues regarding embryonic development, wound healing, and how to target tumor–stroma signaling.
Keywords: Notch signaling, Jagged, Fringe, cell signaling, developmental biology
Abstract
Notch signaling pathway mediates cell-fate determination during embryonic development, wound healing, and tumorigenesis. This pathway is activated when the ligand Delta or the ligand Jagged of one cell interacts with the Notch receptor of its neighboring cell, releasing the Notch Intracellular Domain (NICD) that activates many downstream target genes. NICD affects ligand production asymmetrically––it represses Delta, but activates Jagged. Although the dynamical role of Notch–Jagged signaling remains elusive, it is widely recognized that Notch–Delta signaling behaves as an intercellular toggle switch, giving rise to two distinct fates that neighboring cells adopt––Sender (high ligand, low receptor) and Receiver (low ligand, high receptor). Here, we devise a specific theoretical framework that incorporates both Delta and Jagged in Notch signaling circuit to explore the functional role of Jagged in cell-fate determination. We find that the asymmetric effect of NICD renders the circuit to behave as a three-way switch, giving rise to an additional state––a hybrid Sender/Receiver (medium ligand, medium receptor). This phenotype allows neighboring cells to both send and receive signals, thereby attaining similar fates. We also show that due to the asymmetric effect of the glycosyltransferase Fringe, different outcomes are generated depending on which ligand is dominant: Delta-mediated signaling drives neighboring cells to have an opposite fate; Jagged-mediated signaling drives the cell to maintain a similar fate to that of its neighbor. We elucidate the role of Jagged in cell-fate determination and discuss its possible implications in understanding tumor–stroma cross-talk, which frequently entails Notch–Jagged communication.
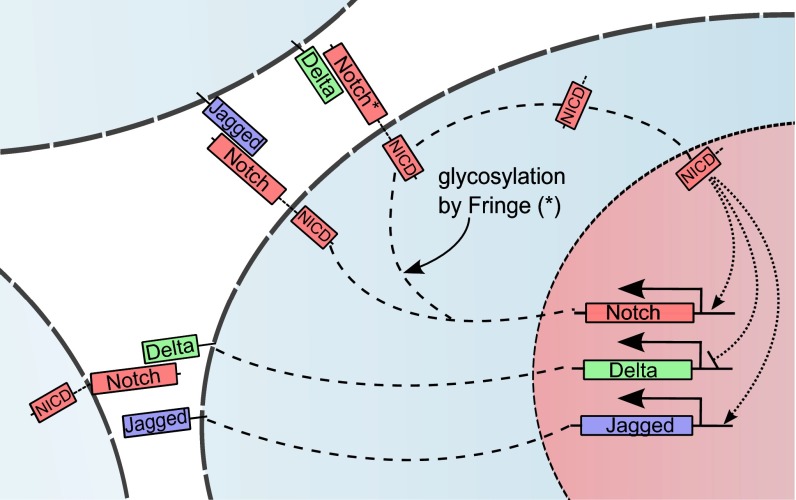
Notch signaling pathway is an evolutionarily conserved mechanism that plays a crucial role in controlling cell-fate differentiation during embryonic development (1, 2). This pathway is often aberrantly activated in many cancers and controls the proliferation and survival of cancer cells, as well as their malignant progression (3). The signaling pathway consists of the Notch transmembrane receptor and its ligands Delta and/or Jagged. The interaction between the receptor and the ligand of the same cell (cis-interaction) leads to the degradation of both proteins, therefore not generating a signal. The interaction between the receptor of one cell with the ligand of a neighboring cell (trans-interaction) leads to the release of the Notch Intracellular Domain (NICD) signal into the cytoplasm. The NICD then enters the nucleus where it associates with the CSL transcription factor complex, resulting in subsequent activation of downstream target genes (1, 2) (Fig. 1).
Fig. 1.
Overview of the intracellular and intercellular Notch signaling pathway. Notch, the transmembrane receptor of one cell, binds to Delta or Jagged, the transmembrane ligands belonging to the neighboring cell. This trans-interaction cleaves the Notch receptor to release NICD. NICD migrates to the nucleus and modulates the transcription of many genes. This modulation indirectly leads to the transcriptional activation of Notch and Jagged and inhibition of Delta. Interaction between Notch receptor and ligands (Delta or Jagged) of the same cell (cis-interaction) leads to the degradation of both the receptor and the ligand. Glycosylation of Notch by Fringe modifies Notch to have higher affinity for binding to Delta and lower affinity for binding to Jagged.
Notch signaling through Jagged and that through Delta have different dynamics because of two elements of asymmetry in the signaling circuit. First, NICD inhibits Delta through its downstream effector Hes1 (4), but activates Jagged both directly (5) and indirectly through miR-200 (see discussion in SI Text, section S1). These modes of regulation effectively create an intercellular double-negative feedback loop between Notch and Delta (6), but an intercellular double-positive feedback loop between Notch and Jagged (5) (Fig. 1). Consequently, Notch–Delta signaling between two cells behaves as a two-way switch: one cell has [high Delta (ligand), low Notch (receptor)] expression on its surface, whereas the other cell has [high Notch (receptor), low Delta (ligand)] on its surface. According to common terminology, the first cell behaves as a Sender (S) and the second one as a Receiver (R). In other words, the Notch–Delta signaling standalone causes the two neighboring cells to acquire opposite fates. This mechanism, known as lateral inhibition, is implicated, for example, in control of neurogenesis in Drosophila and vertebrates (7), and in salt-and-pepper patterns observed during wing vein formation (6). On the other hand, for standalone Notch–Jagged signaling between two cells, Notch and Jagged levels in both cells go hand in hand (high Notch, high Jagged). Therefore, both cells can act as both Receiver (R) and Sender (S)––or the two cells acquire similar fates. This mechanism, known as lateral induction, is implicated, for example, in mammalian inner-ear development (8, 9), control of epidermal stem cell clusters (10), as well as inner cardiac development (11). Therefore, Delta and Jagged affect the collective cell-fate decisions in a group of cells quite differently.
The second asymmetry between signaling through the ligands Delta and Jagged arises due to posttranslational modifications of Notch that modulate the binding of Notch to Delta and to Jagged. Fringe, a glycosyltransferase, can decrease the affinity of Notch to bind to Jagged, but increase the affinity of Notch to bind to Delta (12). Consequently, Fringe creates two distinct Notch populations on the cell surface: one that has comparable binding affinity to both Jagged and Delta, and one that strongly prefers binding to Delta. The effects of these two elements of asymmetry in Notch signaling remain elusive and call for clarification of their corresponding role in cell-fate determination mediated by Notch signaling.
Many experimental and theoretical research efforts have been directed toward understanding the Notch–Delta-dependent cell-fate determination (6, 13–17). In contrast, the role of Notch–Jagged signaling has gained limited research attention despite the recognized role of Jagged in tumorigenesis. For example, overexpression of Jagged has been associated with poor prognosis, at least in breast cancer and prostate cancer (18), thus highlighting the importance of understanding its role in Notch signaling. Other recent studies have shown that Notch signaling can be activated by soluble forms of the ligands Jagged and Delta (19–21). The soluble Jagged and Delta have different effects on tumor progression––soluble Delta inhibits tumor growth (22, 23), whereas soluble Jagged strongly aggravates the malignant progression of cancer. More specifically, Jagged plays an important role in inducing epithelial to mesenchymal transition (EMT) as well as promoting cells to acquire cancer stem cell (CSC) properties (20). Notably, Notch–Jagged signaling also plays a crucial role in angiogenesis (24), cancer metastasis (25), and rapid development of cancer chemotherapy and radiation therapy resistance (26).
Here, we have devised a tractable mathematical framework to evaluate the role of Jagged in cell-fate determination mediated by Notch signaling. We show that the Jagged–Delta asymmetry in Notch signaling can give rise to a Sender–Receiver (S/R) hybrid state, thus rendering the Notch signaling to operate as a three-way switch so that two interacting cells can acquire one of the three states––Sender (S), Receiver (R), and hybrid Sender/Receiver (S/R). More specifically, we demonstrate how including Jagged in the Notch–Delta signaling opens up and maintains a previously unidentified state in which the cells can both send and receive signals––suggesting that Jagged-mediated signaling allows interacting cells to acquire similar fates.
Results
The Theoretical Framework.
To explore the effects of Jagged in cell-fate determination, we generalized earlier theoretical framework devised by Sprinzak et al. (14) by incorporation of Jagged in addition to Delta, and the asymmetric transcription regulation of the ligands by NICD––a transcriptional activator of Jagged and transcriptional repressor of Delta. First, we investigated the model dynamics in the case when Jagged and Delta have similar binding affinity of Notch. Second, we analyzed a further extension of the model in which the asymmetric effect Fringe is included: Fringe increases the Notch–Delta binding affinity and decreases the Notch–Jagged binding affinity.
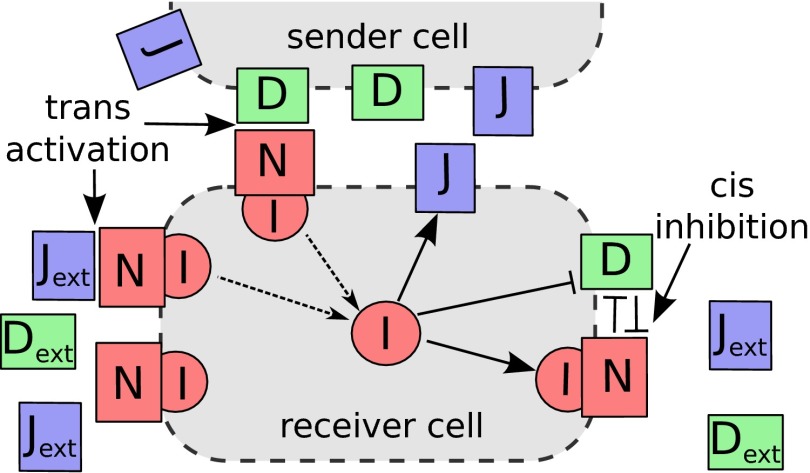
More specifically, within the framework proposed by Sprinzak et al. (14), Notch receptor (N) belonging to one cell can interact with both the ligands of the same cell (D or J)––known as cis-interaction, or with those of the neighboring cell––Jagged or Delta ( or )––known as trans-interaction. The cis-interaction, also referred to as cis-inhibition, causes the degradation of both the interacting proteins. On the other hand, the trans-interaction, also referred to as trans-activation, leads to the cleavage of Notch receptor, which releases NICD (represented as I in the model). Within the framework presented here, in addition to introducing Jagged as an additional element in the signaling circuit, we also include the feedback effects of NICD that indirectly activates Notch and Jagged and represses Delta, thereby creating an asymmetry between Notch–Delta and Notch–Jagged interactions (Fig. 2). The deterministic equations for the dynamics of Notch (N), Delta (D), Jagged (J), and NICD (I) are given by
| [1] |
| [2] |
| [3] |
| [4] |
where γ represents the degradation rate of all three transmembrane proteins Notch, Jagged, and Delta, and the degradation rate of NICD. and are the strengths of cis-inhibition and trans-activation, respectively; and , , and are the production rates of Notch, Delta, and Jagged, respectively. , , and represent the amount of protein available for binding, which can be on the membrane surface of neighboring cells or in a soluble form. Experimental evidence suggests that membrane-bound ligands can generate a stronger signal compared with their soluble forms (27). However, the distinction between these two forms of ligand––membrane-bound and soluble––is not addressed in the following analysis but can be easily incorporated in model by considering their different trans-activation rates (Eqs. S41–S44 in SI Text, section S6). We consider shifted Hill functions (28) to represent the effect of NICD (I) on the production rates of the proteins. Shifted Hill functions are defined as or in simpler notation: if and if , where the weight factor λ represents the fold change in production rate, therefore, for activation, ; for repression, ; and for no effect, (, , and in our model). Note that shifted Hill functions have a constitutive production term. For the case of two interacting cells, the variables , , and should be replaced by N, D, J of the neighboring cell (Eqs. S22–S25 in SI Text, section S3). A detailed discussion of the parameter values can be found in SI Text, section S1, and Table S1. The model shows a good robustness with respect to changes in parameter values as discussed in SI Text, section S5, and Figs. S1 and S2. Temporal dynamics and stochastic simulations are included in SI Text, section S7, and Fig. S3. All of the codes were developed in Python using the PyDSTool (29).
Fig. 2.
Schematic illustration of Notch signaling circuit. NICD (I) is released when the receptor (N) of the receiver cell interacts with the ligand of the sender cell (D or J) or with external ligands in a soluble form ( or )––so-called trans-activation. The released signal activates the expression of N and J, and inhibits the expression of D. The cis-inhibition occurs between the receptor and ligand in the same cell and leads to the degradation of both proteins.
Notch–Delta Circuit: A Two-Cell Toggle Switch.
We proceed to analyze the standalone dynamics of the Notch–Delta signaling by analyzing the reduced model given by Eqs. (5)–(7) below (a reduced version of the model described above):
| [5] |
| [6] |
| [7] |
We analyze two cases of this model: (i) single cell driven by a cell with fixed value of external Delta () and fixed value of external Notch (); (ii) two interacting cells where () and () represent the D and N of the neighboring cell. For case (i), the Notch–Delta circuit is bistable with two possible states: (a) (high Notch, low Delta)––the cell is a Receiver (R) of the ligand D, and (b) (low Notch, high Delta)––the cell is a Sender (S) of the ligand D (Fig. 3A). For case (ii), the ligands D of both cells activate the receptors of the other cell, and the two-cell circuit presents two possible states: the first cell as Receiver and the second cell as Sender (R; S) and vice versa, i.e. (S; R) (Fig. 3B and Fig. S4D). The model for this case of two cells interacting through Notch–Delta is detailed in SI Text, section S3, Eqs. S19–S21.
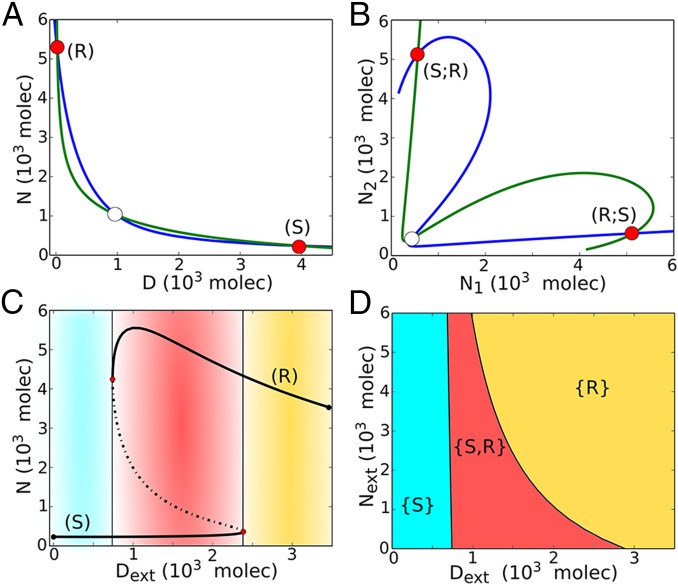
Fig. 3.
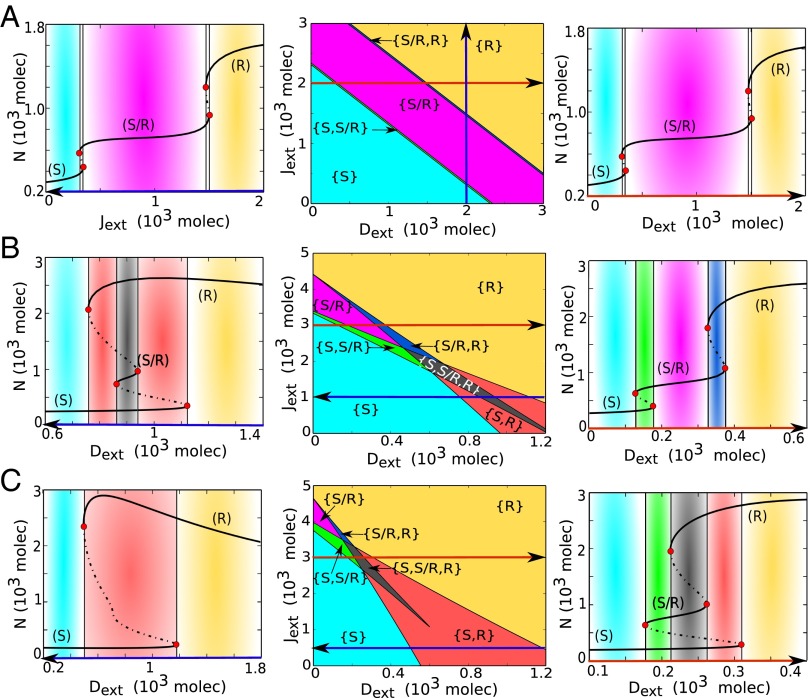
Dynamical system characteristics of the Notch–Delta circuit. (A) Nullclines for the case of one cell interacting with fixed values of external proteins (, ). The blue nullcline is for condition and , and the green nullcline is for condition and (Eqs. 5–7). Unfilled circles represent unstable steady states, whereas red filled circles represent the two stable states–– the Sender (S) and the Receiver (R). (B) Nullclines for the case of two cells interacting with each other through Notch–Delta. The blue nullcline is for condition of all ODEs being set to zero except for and the green nullcline is for condition of all ODEs being set to zero except for (Eqs. S19–S21). Unfilled circle represents unstable steady states, and the red filled circles represent the two stable states––(S; R) and (R; S). (C) Bifurcation, for the one-cell case, of Notch protein levels on the membrane as a function of the number of external Delta for fixed molecules. Starting in the Sender (S) state, i.e., (low Notch, high Delta) (blue region) and increasing the external Delta at some threshold the cell undergoes a transition to the Receiver state, i.e., (high Notch, low Delta) (yellow region). The reverse transition occurs at a different number of proteins that leads to a region of coexistence of both states––Sender and Receiver (red region). Solid curves represent stable steady states, whereas dotted curves represent unstable steady states. (D) Phenotype diagram as a function of external Notch and external Delta for one-cell model. The monostable phase {S} corresponds to the Sender state (low Notch, high Delta) and monostable phase {R} corresponds to the Receiver state (high Notch, low Delta). The bistable phase {S,R} corresponds to a region of coexistence of both states––Sender and Receiver.
Bifurcation and Phase Diagram.
In Fig. 3C, we present a bifurcation diagram when the external Delta () acts as a control parameter––the range of existence of the different Notch–Delta states of a single cell as function of (). We see that for small , the cell behaves as a Sender (S); and for large , the cell behaves as a Receiver (R). We further see the existence of bistability for intermediate levels of ; the cell can either be a Sender (S) or a Receiver (R) (Fig. 3C and Fig. S5A). Next, in Fig. 3D, we present the phase diagram (two-parameter bifurcation diagram) for a single cell driven by two control parameters, the external Notch and the external Delta . Doing so reveals the existence of three distinct phases: (i) monostable Sender {S} phase, (ii) monostable Receiver {R} phase, and (iii) a bistable phase {S,R}, where cells can either be Receiver or Sender. The results indicate that the Notch–Delta circuit behaves as an intercellular mutually inhibitory bistable (two-way) toggle-like switch. As such, this switch drives two Notch–Delta interacting neighboring cells to adopt opposite fates: one cell as a Sender and the other as a Receiver or vice versa. This result is consistent with the mutual inhibition mechanism commonly associated with Delta-mediated Notch signaling (30), also referred to as lateral inhibition. For this reason, the Notch–Delta signaling is critical for generating checkerboard-like patterns as well as sharp boundaries of wing vein formation in the Drosophila wing disc (13), and also in the differentiation of sensory cells (31).
The Ligands’ Asymmetric Transcription Regulation by NICD.
In this section, we study the effect of the ligands' asymmetric transcription regulation by NICD––inhibition of Delta and activation of Jagged. We consider the Notch–Jagged cis-inhibition and trans-activation rate to be the same as the Notch–Delta cis-inhibition and trans-activation rate. The effect of the posttranslational modifications of Notch by Fringe is considered in the next section. We found that the ligands' asymmetric transcription regulation enables the existence of a new Sender/Receiver (S/R) hybrid state in addition to the Sender (S) and Receiver (R) states, thus turning the Notch–Delta–Jagged pathway to act as a three-way switch (Fig. 4A and Fig. S4A).
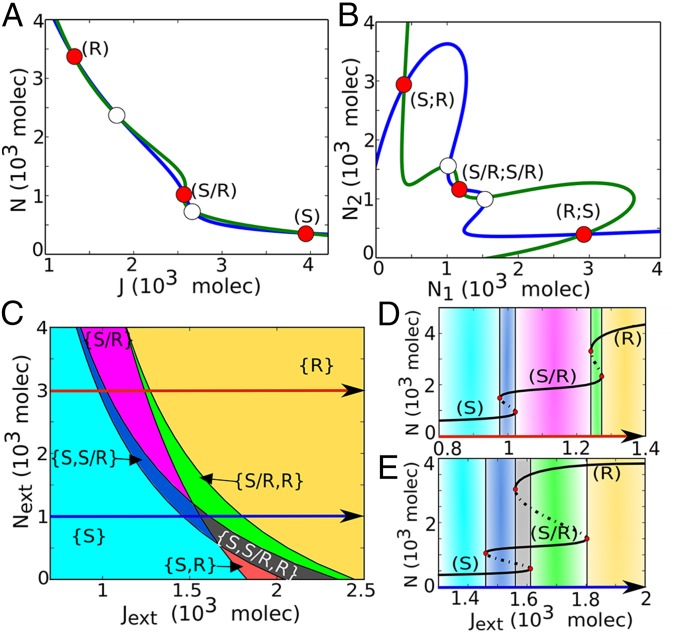
Fig. 4.
Dynamical system characteristics of the Notch–Delta–Jagged circuit. (A) Nullclines for the case of one cell interacting with fixed values of external proteins (, , ). The blue nullcline is for condition , and , and the green nullcline is for condition , and (Eqs. 1–4). The red filled circles represent the three stable steady states––Sender (S), Receiver (R), and hybrid Sender/Receiver (S/R). Unfilled circles represent unstable steady states. (B) Nullclines for the case of two cells interacting with each other through Notch–Delta–Jagged. The blue nullcline is for condition of all ODEs being set to zero except for and the green nullcline is for condition of all ODEs being set to zero except for (Eqs. S22–S25). (C) Phenotype diagram when the one-cell Notch–Delta–Jagged circuit is driven by both the external Notch and external Jagged , for . Each phase, denoted by a different color, corresponds to a different combination of coexisting phases. Same phenotype diagram is obtained when driven by and , for , once Notch is considered to have the same binding affinity as and . (D) Bifurcation of Notch protein levels on the membrane when driven by external Jagged for fixed levels of proteins. This curve shows the existence of the monostable {S/R} phase (pink region) for a large range of external ligands. (E) Same as panel D for proteins. In this case, the hybrid S/R state coexists with other states, i.e., seen only in bistable (blue and green regions) and tristable phases (gray region).
The S/R hybrid state has intermediate levels of both the receptor and the ligands, therefore allowing for bidirectional signaling. In the case of two interacting cells, in this hybrid state, indicated by (S/R; S/R), the two cells have similar intermediate levels of the ligands in contrast with the additional two opposite states [(S; R) and (R; S)] (Fig. 4B and Fig. S4 E and F). In other words, whereas the Notch–Delta signaling enables only opposite fates, (S; R) and (R; S), the Notch–Delta–Jagged signaling enables two similar interacting cells to have similar fates of being in a hybrid state (S/R; S/R). Such similar fate adoption, also known as lateral induction, is a signature of Notch–Jagged signaling. For example, during inner-ear development, lateral induction through Jagged1 in specific regions of the developing otocyst (auditory vesicle) enables the propagation and maintenance of prosensory character in some cells (32). Also, during cardiac development, lateral induction specifies the cells that undergo EMT to form endocardial cushion and heart valves (11). Besides, lateral induction has been implicated in vertebrate somite boundary formation (33) as well as in wing margin development (6, 13).
Phase Diagram.
In Fig. 4C we present the phase diagram (two-parameter bifurcation diagram) for a single cell driven by two control parameters: the external Notch and the external Jagged . When the two ligands are included with asymmetric transcription regulation by NICD, the phase diagram comprises three monostable phases: {S}, {R}, and {S/R}; three phases of coexistence of two phenotypes {S,R}, {S,S/R}, and {S/R,R}; and also a tristable phase showing the coexistence of all three possible states {S,S/R,R} (Fig. 4C). At larger values, we see the hybrid S/R state can exist, for some range of , by itself, i.e., in the monostable {S/R} phase (Fig. 4D and Fig. S5B). However, at smaller values, the hybrid state always coexists with other states in a bistable phase {S, S/R} and {S/R, R}, or in a tristable phase {S, S/R, R} (Fig. 4E and Fig. S5C).
The Effect of Fringe-Mediated Asymmetric Notch–Ligand Binding.
Glycosylation of Notch by Fringe creates additional asymmetry between Delta and Jagged by modulating the binding affinity of the two ligands to Notch; the glycosylated Notch has a higher affinity to bind to Delta, but lower affinity to bind to Jagged (34, 35). To incorporate this mechanism within our framework, we considered two distinct subpopulations of Notch––the one modified by Fringe, and the other unmodified. Because NICD, which is represented in the model by (I), activates Fringe (36), we have taken the fraction of glycosylated Notch (denoting the effect of Fringe on Notch) to increase with (I). This glycosylated Notch has different strengths of cis-inhibition and trans-activation for Delta and for Jagged (37) (see derivation of the model in SI Text, section S2). Thus, while representing effective Notch (sum of glycosylated and unglycosylated Notch), we consider the strengths of cis-inhibition and trans-activation of Notch for Delta and for Jagged to depend on (I). The resulting model for one cell is given by
| [8] |
| [9] |
| [10] |
| [11] |
where and are now functions of the signal NICD given by , where . The shifted Hill function represents the increase of the Fringe effect on the binding asymmetry with the increase of (I) and the parameter represents the increase , decrease of both trans-activation and cis-inhibition rate due to glycosylation. Experimental evidence suggests that and (34, 35), representing the increase of the binding affinity between Notch and Delta, and the decrease of that between Notch and Jagged.
When , the model is the same as considered earlier without any effect of Fringe (Eqs. 1–4). In this case, the Notch–ligand binding has equal affinity for external Jagged and external Delta, as reflected in the symmetry of the phenotype diagram (two-parameter phase diagram) for external Jagged and external Delta (Fig. 5A, Center). The bifurcation diagram for a cell driven by external Delta and by external Jagged presents the same behavior––a large range of the intermediate state (S/R) in a monostable phase (Fig. 5A). However, when the effect of Fringe is incorporated (e.g., and ), the circuit behaves differently. The range of the existence of the four phases containing the hybrid S/R state [the phases {S/R}, {S/R, R}, {S, S/R}, and {S, S/R, R}] increases with the level of external Jagged (Fig. 5B, Center, and Fig. S6). When Notch signaling is mainly mediated by Jagged (high ), both the forward and backward transitions between (S) and (R) states require a transitions into and from the hybrid S/R state as an intermediary step (Fig. 5B, Right and Fig. S5D). Conversely, when the Notch signaling is mainly mediated by Delta (high ), the forward and backward transitions between (S) and (R) do not go through the hybrid state (Fig. 5B, Left and Fig. S5E). When the effect of Fringe is considered to be too strong ( and ), the circuit is mostly bistable and therefore the response of the circuit becomes similar to the case of standalone Notch–Delta signaling (Fig. 5C).
Fig. 5.
Phenotype diagram and bifurcation curves for the one-cell Notch–Delta–Jagged–Fringe circuit. The phenotype diagram shows the different possible phases when the circuit is driven by variable levels of both external Jagged and external Delta. (A) Phenotype diagram (Center) for (no Fringe effect). In this case, the circuit response to external Jagged and external Delta is symmetric. Bifurcation curve of Notch protein levels with respect to varying external Jagged values (Left) for fixed and molecules and (Right) bifurcation curve with respect to varying external Delta values for fixed and molecules. (B) Phenotype diagram (Center) for and (intermediate effect of Fringe). Bifurcation curves of Notch protein levels in response to varying for and molecules (Left), i.e., Notch signaling mainly mediated through Delta and for fixed and molecules (Right), i.e., Notch signaling mainly mediated through Jagged. (C) Phenotype diagram (Center) for and (very strong effect of Fringe). Bifurcation curves of Notch protein levels in response to for fixed and molecules (Left), in which the hybrid S/R state no longer exists, and the circuit behaves like a bistable toggle switch similar to the circuit considering Notch–Delta only, and for fixed and molecules (Right), in which the hybrid S/R state can be observed to coexist with other states (green and gray regions). Phenotype diagrams for are presented in Fig. S7.
The results above suggest that signaling through Jagged has an important role in maintaining the hybrid Sender/Receiver (S/R) state, and that Jagged makes it much more likely that transition from Sender (S) to Receiver (R) and vice versa happens through the hybrid (S/R) state.
The Effect of Delta–Jagged Asymmetry on the Cell–Cell Fate Modulation.
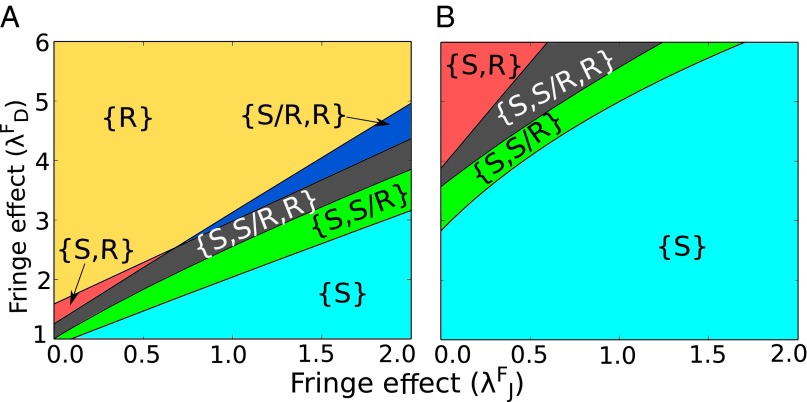
Notch signaling in mammals is mediated through four types of Notch (Notch 1–4) and three types of Fringe (lunatic, manic, and radical Fringe) (38). Experimental evidence suggests that most Fringe proteins act with different types of Notch, possibly leading to different forms of glycosylated Notch, thereby expanding the repertoire of responses that the Notch signaling system can mediate (34, 35). Within our framework, different modulations of Notch by Fringe can be represented by different values of the parameters and , which represent either the increase or decrease of the cis-inhibition and trans-activation rates. Most experimental evidence suggests that Fringe increases the signaling mediated by Delta and decreases the signaling mediated by Jagged, resulting in and (34, 35). The phenotype diagram when the circuit is driven by different values of and presents the response of the circuit for different combinations of Fringe modulations (Fig. 6).
Fig. 6.
Phenotype diagram of the Notch–Delta–Jagged–Fringe model when the circuit is driven by different values of the Fringe modulation for Notch–Delta interaction and Notch–Jagged interaction . In all curves the external signal represents cells in the Sender (S) state––low concentration of Notch and high concentration of ligands. Each figure represents a different combination of the number of external ligands (in number of proteins available to binding). (A) and molecules. (B) and molecules.
Because Fringe is activated by NICD (36), its effect is dominant in cells with high number of Notch molecules [Receiver (R) state] that cleave to form NICD. Therefore, to analyze the effect of Fringe on Notch–Delta–Jagged signaling, we choose the external signal to the cell to be composed mainly of ligands (, ) and low values of ; i.e., the external signal can be considered equivalent to a Sender (S) cell. Two such different combinations are chosen: (high , low ) and (low , high ) (Fig. 6). In the case of (high , low ) and at and , i.e., when the external signal is mostly Delta, and Fringe increases the affinity of Notch for Delta, and decreases that for Jagged, the cell is mostly in monostable phase of the Receiver (R) state, or, in other words, the cell attains the opposite fate as that of a cell representing the external signals (Fig. 6A). However, when the external signal is mainly Jagged, i.e., in (low , high ), at smaller values of and , i.e., the effect of Fringe is not very pronounced; the cell is mostly in the monostable phase of the Sender (S) state (Fig. 6B). Therefore, the cell attains a fate similar to the cell represented by the external signal. These results suggest that while signaling through the Notch–Delta circuit, the two cells attain opposite fates; however, when signaling through the Notch–Jagged circuit, the two cells attain similar fates, thereby suggesting that Jagged helps neighboring cells to maintain similar fates.
Ligand Production Rates Control Tissue Level Patterning.
As mentioned earlier, Notch–Delta interactions lead to lateral inhibition––neighboring cells adopt alternate fates––Sender (S) and Receiver (R) (13, 14, 39, 40). Notch–Jagged interactions lead to lateral induction (41, 42)––neighboring cells adopt similar fates––both of them can simultaneously send and receive the signal–Sender/Receiver (S/R) state. Although these mechanisms have been well-studied individually, the tissue level patterns that might emerge when both mechanisms act simultaneously have not been explored.
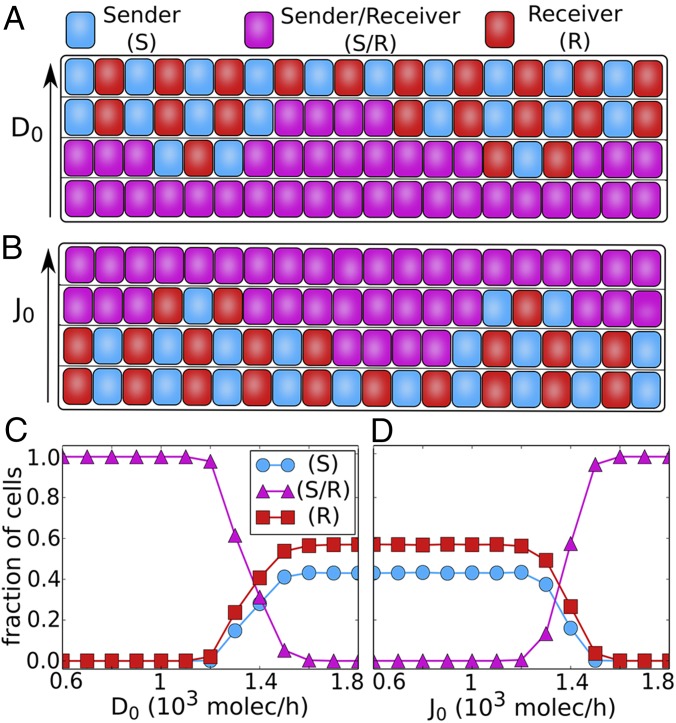
To address this issue, we simulate a one-dimensional layer of cells interacting via Notch pathway, for different values of and ––Delta and Jagged production rates, respectively. Our results show that when the relative production rate of Delta is increased, the so-called “salt-and-pepper” pattern (or alternate fate pattern) at a tissue level begins to emerge (Fig. 7 A and C and Fig. S8). On the other hand, when the relative production rate of Jagged is increased, the salt-and-pepper pattern is disrupted, and the cells begin to adopt similar fates where they can both send and receive the signal (Fig. 7 B and D and Fig. S8).
Fig. 7.
Patterning at the tissue level. (A and B) Representation of a 1D layer of 20 interacting cells. (A) The increase of the production of Delta leads to the formation of alternate patterns in which neighbor cells alternate between Sender and Receiver. (B) The increase of the production of Jagged leads all of the cells to the hybrid (S/R) state, therefore losing the alternate (S) and (R) pattern. (C and D) Average of the fraction of cells in (S), (S/R), or (R) state as a function of ligand production. The averages were taken over 100 simulations of a 1D layer of 100 interacting cells with periodic boundary condition. (C) Fraction of cells in (S), (S/R), or (R) state as a function of the production of Delta. (D) Fraction of cells in (S), (S/R), or (R) state as a function of the production of Jagged. The states of the cells are defined according to the amount of signaling (I): Sender , Sender/Receiver , and Receiver . For this figure, we used .
Our results are consistent with the patterns observed experimentally when both ligands are produced at different rates. For example, during hypoxia-mediated angiogenesis, increase in the concentration of vascular endothelial growth factor increases the production of Delta, thereby causing some cells to adopt a tip fate––those with (high Delta, low Notch). Consequently, the remaining cells adopt a stalk fate––(low Delta, high Notch). It may be noted that in this physiological context, the cells do not necessarily adopt a canonical salt-and-pepper pattern, rather two tip cells might be separated by a few stalk cells, the number of which is determined by Jagged (43). As another example, the inflammatory factors such as TNF-α can lead to increased Jagged production and decreased Delta production (43), therefore driving the cells to a similar fate––hybrid Sender/Receiver (S/R)––that can promote bidirectional communication between tumor and stroma, a context where inflammation often plays a key role.
Discussion
Notch pathway plays crucial roles during embryonic development (1, 2) and also during tumor progression and metastasis (25). Whereas Notch–Delta signaling has been extensively studied both theoretically and experimentally (6, 13–17) and is well understood, the role of Jagged in the Notch–Delta–Jagged signaling is still elusive. Here, we introduced a specially designed theoretical framework to study Notch signaling through both Delta and Jagged which incorporates the effect of the asymmetries between these two key ligands. An earlier attempt included both Delta and Jagged in Notch signaling system (9). Although it explains some of their own experimental results, it does not include some fundamental features of Notch signaling, such as cis-inhibition between Notch and Delta (14), cis-inhibition between Notch and Jagged (37), and the effect of glycosyltransferase Fringe that causes an asymmetry between Notch–Delta and Notch–Jagged signaling (34, 35). Further, their model also does not discriminate between the transmembrane receptor Notch and the internalized signal NICD.
Our results confirmed that Notch–Delta alone allows only two states: Sender (S) or Receiver (R), which is consistent with previous studies (6, 13, 14, 17). Our key findings are that due to the Delta–Jagged asymmetry, the Notch signaling through both Delta and Jagged gives rise to a hybrid Sender/Receiver (S/R) state in addition to Sender and Receiver states. Sender (S) cells have high levels of ligands (Delta and Jagged) and low levels of receptor (Notch) on their surface, and Receiver (R) cells have high levels of receptor and low levels of ligands on their surface. The hybrid S/R cells have intermediate levels of both the receptor and ligands, therefore allowing them to both send and receive signals. Alternate arrangements of Sender and Receiver cells have been observed in checkerboard-like or salt-and-pepper pattern formation (30, 31). However, direct measurements of both Notch and ligands are needed in cells to identify the hybrid S/R cells.
The two-cell model explores the canonical signaling between two cells. In our one-cell model, we take the level of the external ligands Jagged and Delta (, ) as control parameters that represent fixed levels of Delta and Jagged on a neighboring cell. In the case of Notch–Delta–Jagged between two neighboring cells, (, ) for each cell represent the values of Delta and Jagged on the neighboring one.
We note that (, ) can also represent external soluble ligands. However, currently the mechanism of receptor activation by soluble ligands is not clear and is still debated. Some studies suggest that mechanical pulling force is essential to activate proteolysis and the release of the signal (NICD) (44), and because soluble forms of the ligands tend to lack this pulling force, they are expected to inhibit signaling (45). Conversely, other studies indicate that other mechanisms such as ligand multimerization (46) can furnish sufficient mechanical leverage for receptor activation (12). Notwithstanding the incomplete understanding of how soluble ligands activate Notch, they play crucial roles in various contexts such as de novo generation of regulatory T cells (19), differentiation of adipocyte progenitor (47), hematopoietic progenitor (48), and neural crest stem cells (49).
Soluble Jagged1 has been specifically implicated in mediating long-distance communication between tumor cells and stromal cells. Jagged1 can be secreted by endothelial cells that can activate Notch signaling in cancer cells, inducing them to gain migratory and invasive characteristics by undergoing partial or complete EMT (50). Jagged1 can also induce the expression of NF-κB (51), which can increase the population of CSCs and further increase the secretion of Jagged1 (52), suggesting a wave-like mechanism in the tumor microenvironment to increase the production and maintenance of therapy-resistant CSCs. Future theoretical studies of these circuits hold promise for appreciating the key role of soluble Jagged1 in mediating two interlinked and clinically insuperable facets of cancer––metastasis (as a result of cells undergoing EMT) and tumor relapse (as a result of expanded CSC pool).
Not only soluble Jagged1, but also transmembrane Jagged1 mediates tumor progression in several ways, and has been proposed to be a therapeutic target (53). Notch–Jagged signaling plays a crucial role in the metastasis of breast cancer cells to bone, where prostate cancer cells expressing Jagged1 communicate with Notch-expressing osteoclasts to “home” in the bone (25). Also, overexpression of Jagged1 on cancer cells can trigger Notch activation in neighboring endothelial cells (which can possibly secrete more soluble Jagged1), promoting sprouting tumor angiogenesis (54) and thereby tumor growth. Consistent with their protumor roles, high levels of Notch and Jagged1 in cancer cells often correlate with poor patient survival (53).
We show that the hybrid S/R state is enabled only after including Jagged in the model. We expect that this hybrid state plays an absolutely critical role in mediating communication between cells that have undergone partial EMT and move collectively (28, 55, 56). Notch signaling observed during wound healing (57), a typical case of partial EMT, supports this hypothesis. Specifically, we expect that Notch–Jagged signaling between these cells helps them to maintain that otherwise metastable hybrid epithelial/mesenchymal (E/M) phenotype. As has been recently observed in clusters of circulating tumor cells (CTCs), these hybrid E/M cells mediate tumor aggression and invasion (58), and can have more metastatic potential than the CTCs moving individually (59, 60). Future theoretical studies should investigate the coupling of EMT and Notch–Delta–Jagged signaling to explore this hypothesis.
Although our model provides a fresh theoretical framework to investigate the effect of both Delta and Jagged in the Notch signaling system, it is based on a well-mixed ordinary differential equation (ODE) approximation, ignoring most spatial effects that can be useful, for example, to understand the formation of sharply defined bands of Notch signaling that occur in Drosophila wing vein system (6). Other limitations of our model include: no distinction between soluble and membrane-bound ligands, no time delay between production of Fringe and its action on Notch, and grouping the different members of the family of Notch, Delta, Jagged, and Fringe into one variable. This grouping restricts understanding the context-specific function of different family members, for example, lunatic Fringe vs. radical Fringe (37).
To conclude, we present, to our knowledge, the first step toward including the role of Jagged in cell-fate determination. Jagged-mediated signaling indicates an evolutionary need to implicate different repertoires of responses in cell–cell communication, and has been shown to be critical in mammalian embryonic development as well as tumor progression. A better understanding of Notch–Delta–Jagged signaling, which is affected by various signals in the tumor microenvironment (43), can provide valuable clues how to target cancer survival by interfering with the tumor–stroma cross-talk.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (NSF) (Grants PHY-1427654 and NSF-MCB-1214457) and by the Cancer Prevention and Research Institute of Texas (CPRIT). M.B. was also supported by FAPESP Grant 2013/14438-8. M.L. has a training fellowship from the Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia (also supported by CPRIT Grant RP140113). C.C. was also supported by NSF Grants CHE 1265929 and 1152344 and Welch Foundation Grant C1570. E.B.-J. was also supported by the Tauber Family Funds and the Maguy–Glass Chair in Physics of Complex Systems.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416287112/-/DCSupplemental.
References
- 1.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 2.Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 3.Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28(3):339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 4.Shimojo H, Ohtsuka T, Kageyama R. Dynamic expression of notch signaling genes in neural stem/progenitor cells. Front Neurosci. 2011;5:78. doi: 10.3389/fnins.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manderfield LJ, et al. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125(2):314–323. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaya O, Sprinzak D. From Notch signaling to fine-grained patterning: Modeling meets experiments. Curr Opin Genet Dev. 2011;21(6):732–739. doi: 10.1016/j.gde.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Beatus P, Lendahl U. Notch and neurogenesis. J Neurosci Res. 1998;54(2):125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovic J, et al. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development. 2014;141(11):2313–2324. doi: 10.1242/dev.108100. [DOI] [PubMed] [Google Scholar]
- 10.Savill NJ, Sherratt JA. Control of epidermal stem cell clusters by Notch-mediated lateral induction. Dev Biol. 2003;258(1):141–153. doi: 10.1016/s0012-1606(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman LA, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprinzak D, Lakhanpal A, LeBon L, Garcia-Ojalvo J, Elowitz MB. Mutual inactivation of Notch receptors and ligands facilitates developmental patterning. PLOS Comput Biol. 2011;7(6):e1002069. doi: 10.1371/journal.pcbi.1002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprinzak D, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465(7294):86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collier JR, Monk NA, Maini PK, Lewis JH. Pattern formation by lateral inhibition with feedback: A mathematical model of delta-notch intercellular signalling. J Theor Biol. 1996;183(4):429–446. doi: 10.1006/jtbi.1996.0233. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi Y, Iwasa Y, Hirashima T. Mathematical study of the role of Delta/Notch lateral inhibition during primary branching of Drosophila trachea development. Biophys J. 2012;103(12):2549–2559. doi: 10.1016/j.bpj.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Liu K, Chen L, Aihara K. Neural fate decisions mediated by trans-activation and cis-inhibition in Notch signaling. Bioinformatics. 2011;27(22):3158–3165. doi: 10.1093/bioinformatics/btr551. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279(1):8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campese AF, et al. 2014. Mouse Sertoli cells sustain de novo generation of regulatory T Cells by triggering the Notch pathway through soluble JAGGED1. Biol Reprod 90(3):53.
- 20.Lu J, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23(2):171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urs S, et al. Soluble forms of the Notch ligands Delta1 and Jagged1 promote in vivo tumorigenicity in NIH3T3 fibroblasts with distinct phenotypes. Am J Pathol. 2008;173(3):865–878. doi: 10.2353/ajpath.2008.080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, et al. Resuscitating cancer immunosurveillance: Selective stimulation of DLL1-Notch signaling in T cells rescues T-cell function and inhibits tumor growth. Cancer Res. 2011;71(19):6122–6131. doi: 10.1158/0008-5472.CAN-10-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JL, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67(23):11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 24.Phng LK, Gerhardt H. Angiogenesis: A team effort coordinated by notch. Dev Cell. 2009;16(2):196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer. 2011;105(12):1805–1810. doi: 10.1038/bjc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamdje AN, et al. Role of stromal cell-mediated Notch signaling in CLL resistance to chemotherapy. Blood Cancer J. 2012;2(5):e73. doi: 10.1038/bcj.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narui Y, Salaita K. Membrane tethered delta activates notch and reveals a role for spatio-mechanical regulation of the signaling pathway. Biophys J. 2013;105(12):2655–2665. doi: 10.1016/j.bpj.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci USA. 2013;110(45):18144–18149. doi: 10.1073/pnas.1318192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clewley R. Hybrid models and biological model reduction with PyDSTool. PLOS Comput Biol. 2012;8(8):e1002628. doi: 10.1371/journal.pcbi.1002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barad O, Hornstein E, Barkai N. Robust selection of sensory organ precursors by the Notch-Delta pathway. Curr Opin Cell Biol. 2011;23(6):663–667. doi: 10.1016/j.ceb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kiernan AE. Notch signaling during cell fate determination in the inner ear. Semin Cell Dev Biol. 2013;24(5):470–479. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2(1):e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9(6):583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- 34.Hicks C, et al. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol. 2000;2(8):515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, et al. Manic fringe and lunatic fringe modify different sites of the Notch2 extracellular region, resulting in different signaling modulation. J Biol Chem. 2001;276(28):25753–25758. doi: 10.1074/jbc.M103473200. [DOI] [PubMed] [Google Scholar]
- 36.Morales AV, Yasuda Y, Ish-Horowicz D. Periodic Lunatic fringe expression is controlled during segmentation by a cyclic transcriptional enhancer responsive to notch signaling. Dev Cell. 2002;3(1):63–74. doi: 10.1016/s1534-5807(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 37.LeBon L, Lee TV, Sprinzak D, Jafar-Nejad H, Elowitz MB. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. eLife. 2014;3:e02950. doi: 10.7554/eLife.02950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tien AC, Rajan A, Bellen HJ. A Notch updated. J Cell Biol. 2009;184(5):621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Formosa-Jordan P, Ibañes M. Competition in notch signaling with cis enriches cell fate decisions. PLoS ONE. 2014;9(4):e95744. doi: 10.1371/journal.pone.0095744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JS, Gumbayan AM, Zeller RW, Mahaffy JM. An expanded Notch-Delta model exhibiting long-range patterning and incorporating MicroRNA regulation. PLOS Comput Biol. 2014;10(6):e1003655. doi: 10.1371/journal.pcbi.1003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Back W, Zhou JX, Brusch L. On the role of lateral stabilization during early patterning in the pancreas. J R Soc Interface. 2013;10(79):20120766. doi: 10.1098/rsif.2012.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owen MR, Sherratt JA, Wearing HJ. Lateral induction by juxtacrine signaling is a new mechanism for pattern formation. Dev Biol. 2000;217(1):54–61. doi: 10.1006/dbio.1999.9536. [DOI] [PubMed] [Google Scholar]
- 43.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin Cell Dev Biol. 2012;23(4):429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small D, et al. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276(34):32022–32030. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- 46.Hicks C, et al. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J Neurosci Res. 2002;68(6):655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- 47.Urs S, et al. Effect of soluble Jagged1-mediated inhibition of Notch signaling on proliferation and differentiation of an adipocyte progenitor cell model. Adipocyte. 2012;1(1):46–57. doi: 10.4161/adip.19186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han W, Ye Q, Moore MA. A soluble form of human Delta-like-1 inhibits differentiation of hematopoietic progenitor cells. Blood. 2000;95(5):1616–1625. [PubMed] [Google Scholar]
- 49.Morrison SJ, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101(5):499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 50.Bao B, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Wang Z, et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109(4):726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto M, et al. NF-κB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat Commun. 2013;4:2229. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front Oncol. 2014;4:254. doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon CH, et al. High glucose-induced jagged 1 in endothelial cells disturbs notch signaling for angiogenesis: A novel mechanism of diabetic vasculopathy. J Mol Cell Cardiol. 2014;69:52–66. doi: 10.1016/j.yjmcc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Lu M, et al. Tristability in cancer-associated microRNA-TF chimera toggle switch. J Phys Chem B. 2013;117(42):13164–13174. doi: 10.1021/jp403156m. [DOI] [PubMed] [Google Scholar]
- 56.Lu M, Jolly MK, Onuchic J, Ben-Jacob E. Toward decoding the principles of cancer metastasis circuits. Cancer Res. 2014;74(17):4574–4587. doi: 10.1158/0008-5472.CAN-13-3367. [DOI] [PubMed] [Google Scholar]
- 57.Chigurupati S, et al. Involvement of notch signaling in wound healing. PLoS ONE. 2007;2(11):e1167. doi: 10.1371/journal.pone.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jolly MK, et al. Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J R Soc Interface. 2014;11(101):20140962. doi: 10.1098/rsif.2014.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.