Abstract
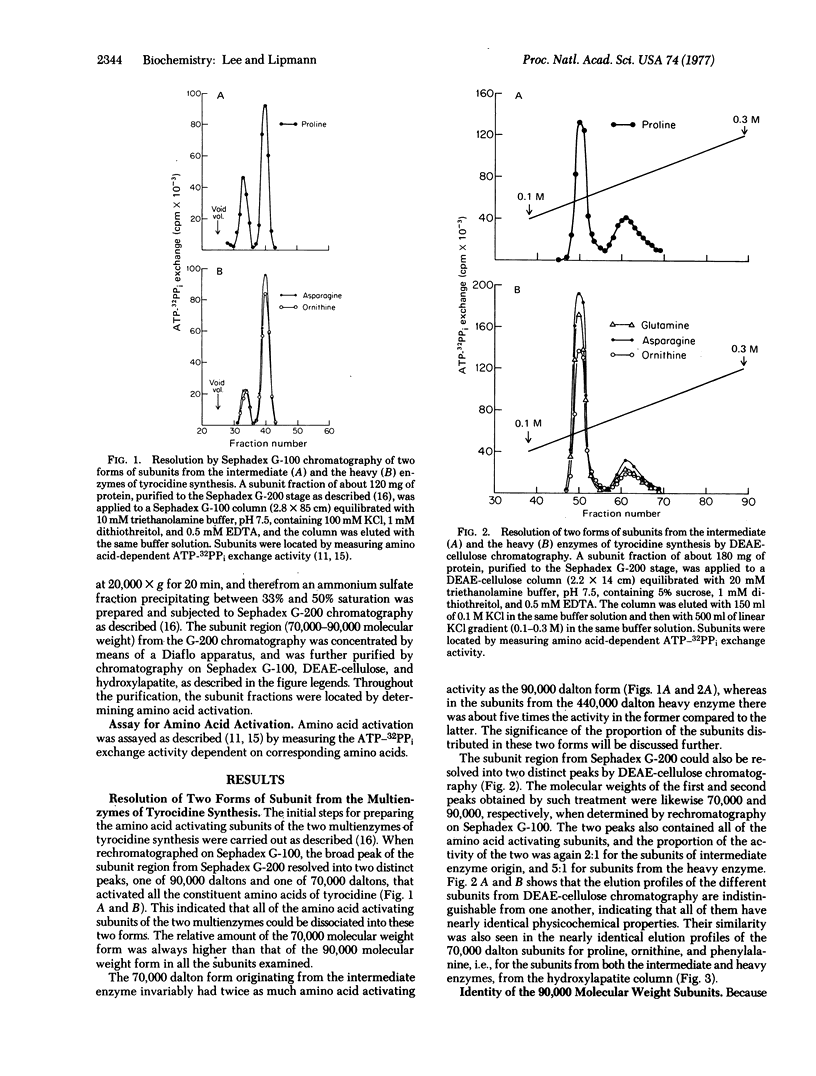
Dissociation of the multienzymes of tyrocidine synthesis by prolonged incubation of crude extracts of Bacillus brevis (Dubos strain, ATCC 8185) has yielded, on Sephadex G-100 chromatography, two fractions of amino acid activating subunits, a larger one of 70,000 daltons and a smaller one of 90,000 daltons; the latter was a complex consisting of the 70,000 dalton subunit and the pantetheine-carrying protein of about 20,000 daltons. When it dissociated, the intermediate enzyme, which activates three amino acids, contained two-thirds of the subunits in the 70,000 dalton and one-third in the 90,000 dalton fraction; the heavy enzyme, which activates six amino acids, contained five-sixths of the subunits in the former fraction and one-sixth in the latter. Both fractions showed ATP-PPi exchange with all amino acids that are activated by the respective polyenzymes. With proline as an example, the 70,000 dalton subunit exhibited a single low-affinity binding site, which should correspond to the peripheral thiol acceptor site, whereas the 90,000 dalton subunit showed both a low-affinity binding site and an additional high-affinity site for proline; the high-affinity site is attributed to the pantetheine present on the pantetheine-carrying protein, and suggests that amino acids are translocated from the peripheral SH to the pantetheine-carrying moiety during chain elongation. This was confirmed by the observation that the 90,000 dalton complex, when incubated with the light enzyme in the presence of phenylalanine and proline, produced DPhe-Pro dipeptide that cyclized into DPhe-Pro diketopiperazine, but the 70,000 dalton activating subunit, when similarly incubated, did not. After subunit dissociation, however, no further elongation occurred after the transfer from phenylalanine to proline.
Keywords: antibiotic peptides, nonribosomal peptide synthesis, peptide bond formation, multienzymes
Full text
PDF
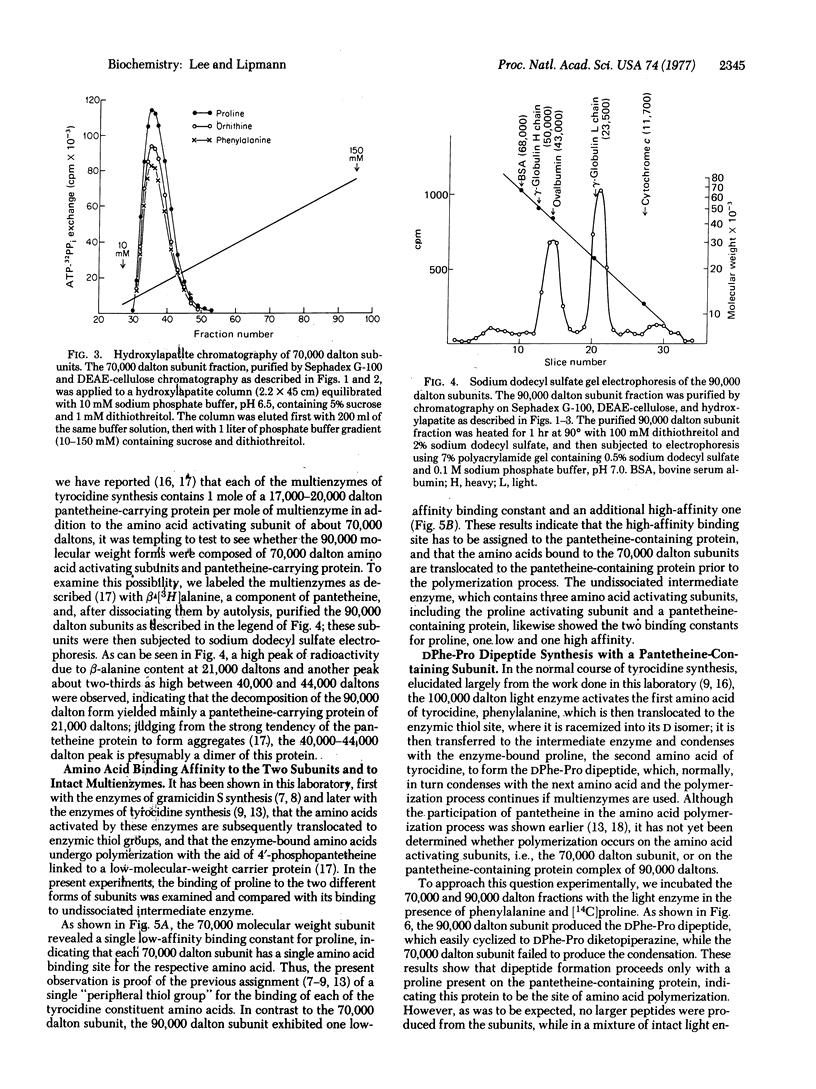

Images in this article
Selected References
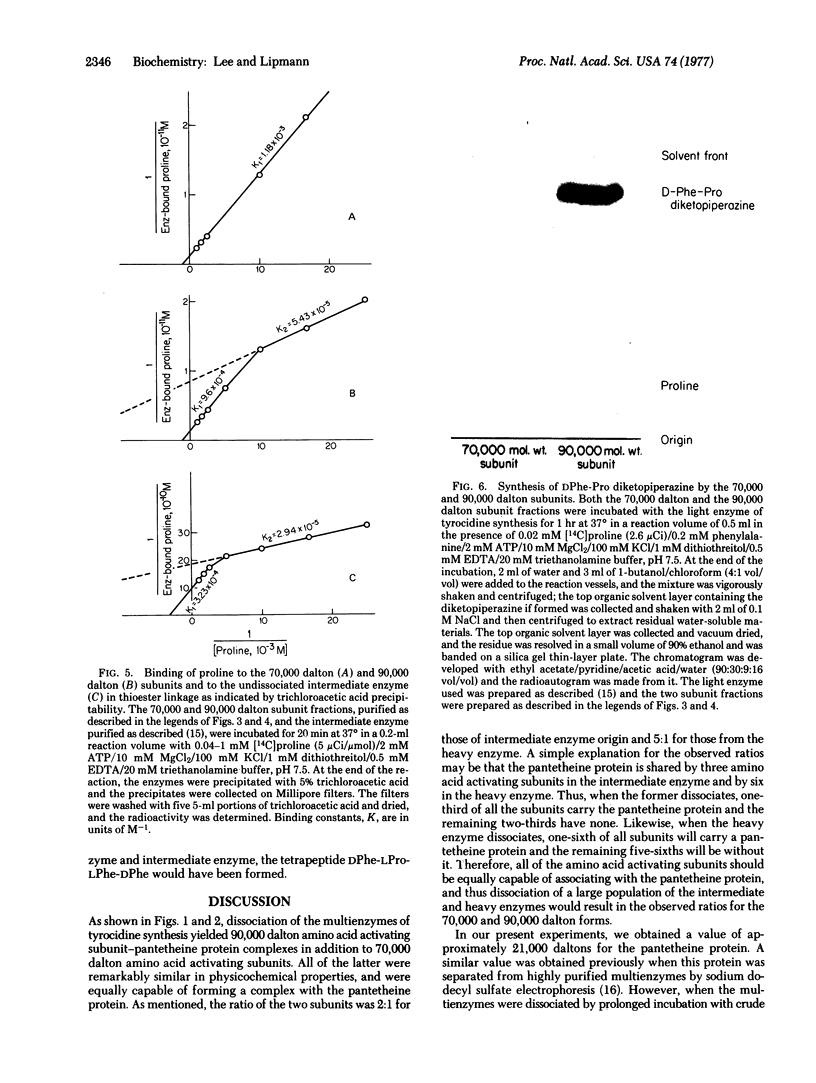
These references are in PubMed. This may not be the complete list of references from this article.
- Fall R. R., Glaser M., Vagelos P. R. Acetyl coenzyme A carbosylase. Circular dichroism studies of Escherichia coli biotin carboxyl carrier protein. J Biol Chem. 1976 Apr 10;251(7):2063–2069. [PubMed] [Google Scholar]
- Froyshov O., Laland S. G. On the biosynthesis of bacitracin by a soluble enzyme complex from Bacillus licheniformis. Eur J Biochem. 1974 Jul 15;46(2):235–242. doi: 10.1111/j.1432-1033.1974.tb03616.x. [DOI] [PubMed] [Google Scholar]
- Gevers W., Kleinkauf H., Lipmann F. Peptidyl transfers in gramicidin S bisoynthesis from enzyme-bound thioester intermediates. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1335–1342. doi: 10.1073/pnas.63.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Yamada M., Tomino S., Kurahashi K. The role of two complementary fractions of gramicidin S synthesizing enzyme system. J Biochem. 1968 Aug;64(2):259–261. doi: 10.1093/oxfordjournals.jbchem.a128888. [DOI] [PubMed] [Google Scholar]
- Jayaraman K., Monteal J., Paulus H. L-Alpha,gamma-diaminobutyrate-activating enzyme from Bacillus polymyxa. Properties and distribution. Biochim Biophys Acta. 1969;185(2):447–457. doi: 10.1016/0005-2744(69)90437-9. [DOI] [PubMed] [Google Scholar]
- Kambe M., Sakamoto Y., Kurahashi K. Biosynthesis of tyrocidine by a cell-free enzyme system of Bacillus brevis ATCC 8185. IV. Further separation of component II into two fractions. J Biochem. 1971 Jun;69(6):1131–1133. doi: 10.1093/oxfordjournals.jbchem.a129567. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., Gevers W., Lipmann F. Interrelation between activation and polymerization in gramicidin S biosynthesis. Proc Natl Acad Sci U S A. 1969 Jan;62(1):226–233. doi: 10.1073/pnas.62.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf H., Roskoski R., Jr, Lipmann F. Pantetheine-linked peptide intermediates in gramicidin S and tyrocidine biosynthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2069–2072. doi: 10.1073/pnas.68.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland S. G., Froyshov O., Gilhuus-Moe C., Zimmer T. L. Gramicidin S synthetase, an enzyme with an unusually large number of catalytic functions. Nat New Biol. 1972 Sep 13;239(89):43–44. doi: 10.1038/newbio239043a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Isolation of a peptidyl-pantetheine-protein from tyrocidine-synthesizing polyenzymes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):607–611. doi: 10.1073/pnas.71.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. G., Littau V., Lipmann F. The relation between sporulation and the induction of antibiotic synthesis and of amino acid uptake in Bacillus brevis. J Cell Biol. 1975 Aug;66(2):233–242. doi: 10.1083/jcb.66.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. G., Roskoski R., Jr, Bauer K., Lipmann F. Purification of the polyenzymes responsible for tyrocidine synthesis and their dissociation into subunits. Biochemistry. 1973 Jan 30;12(3):398–405. doi: 10.1021/bi00727a006. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr, Gevers W., Kleinkauf H., Lipmann F. Tyrocidine biosynthesis by three complementary fractions from Bacillus brevis (ATCC 8185). Biochemistry. 1970 Dec 8;9(25):4839–4845. doi: 10.1021/bi00827a002. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr, Ryan G., Kleinkauf H., Gevers W., Lipmann F. Polypeptide biosynthesis form thioesters of amino acids. Arch Biochem Biophys. 1971 Apr;143(2):485–492. doi: 10.1016/0003-9861(71)90233-5. [DOI] [PubMed] [Google Scholar]
- Tomino S., Yamada M., Itoh H., Kurahashik Cell-free synthesis of gramicidin S. Biochemistry. 1967 Aug;6(8):2552–2560. doi: 10.1021/bi00860a037. [DOI] [PubMed] [Google Scholar]