Abstract
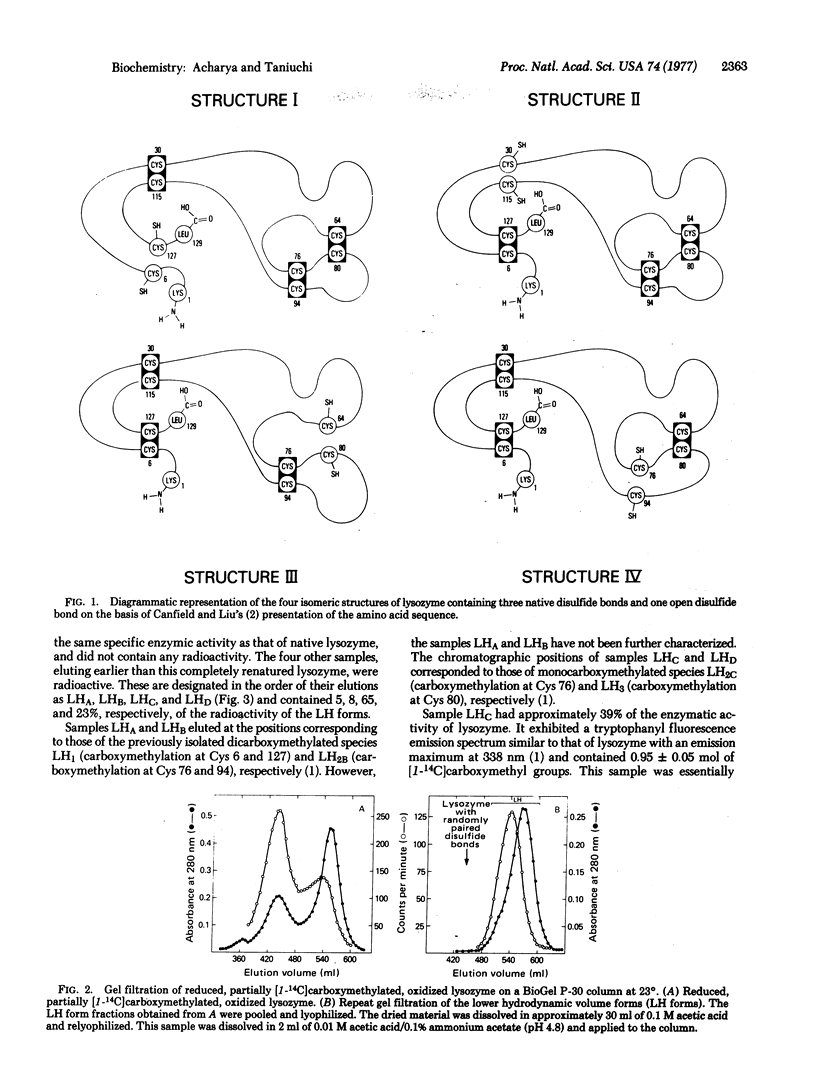
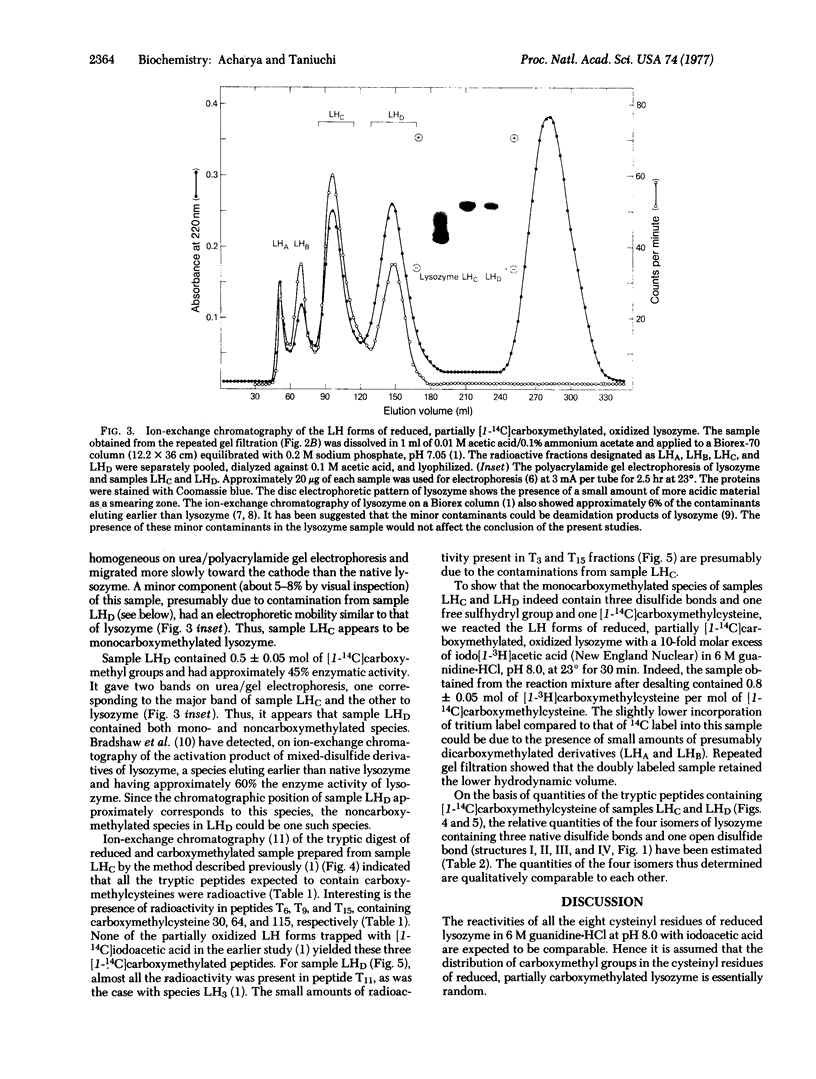
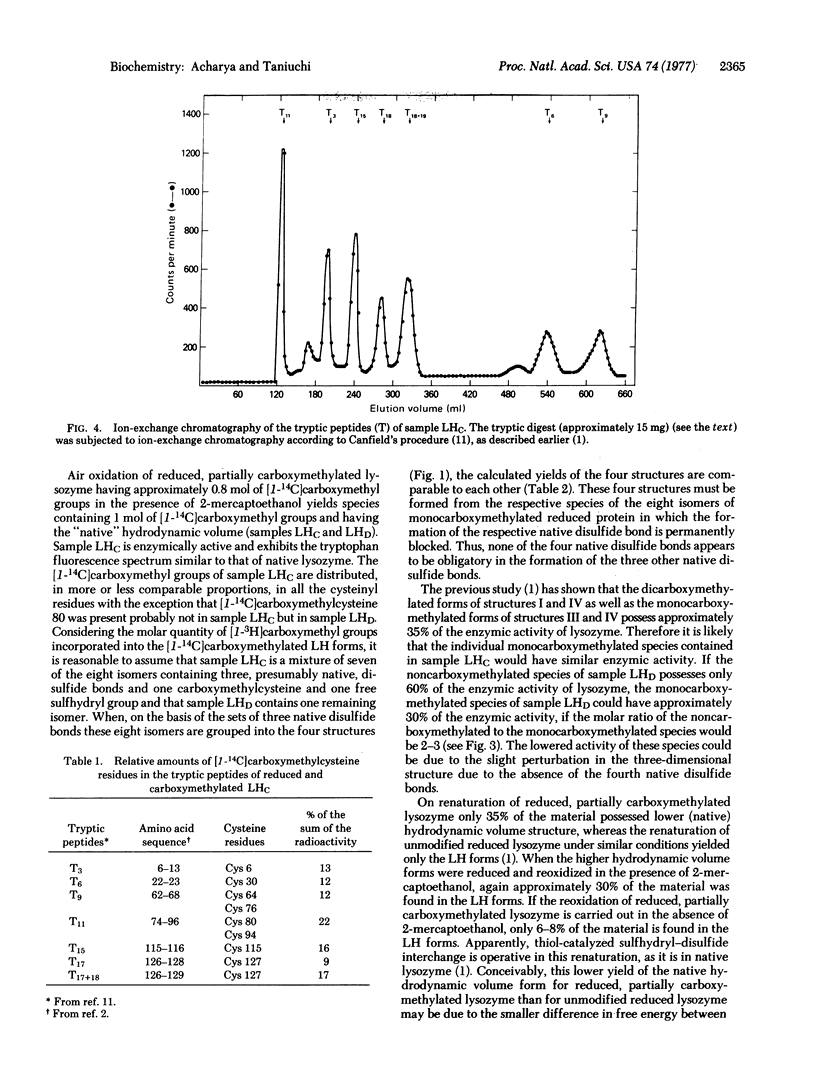
Reduced partially carboxymethylated hen egg white lysozyme (mucopeptide N-acetylmuramoylhydrolase; EC 3.2.1.17) (approximately 0.8 mol of [1-14C]carboxymethyl groups) was air oxidized at pH 8.0 and 37° in the presence of 1.5 mM 2-mercaptoethanol for 36 hr. Gel filtration of this product gave the lower (native) and higher hydrodynamic volume forms, both containing radioactivity (approximately 35 and 65%, respectively). Ion exchange chromatography of the lower hydrodynamic volume forms yielded renatured lysozyme, two major radioactive samples (LHC and LHD) eluting at the positions of monocarboxymethylated lysozyme, and two minor radioactive samples eluting at the positions of dicarboxymethylated lysozyme. Sample LHC (approximately 23% of the radioactivity) was essentially homogeneous with respect to electrophoretic mobility, exhibited approximately 39% of the enzymic activity of lysozyme, and contained 0.95 mol of [14C]carboxymethyl groups. Sample LHD (approximately 8% of the radioactivity) was also enzymically active and contained approximately 0.5 mol of [14C]carboxymethyl groups; this low value is apparently due to contamination of noncarboxymethylated species. The radioactive tryptic peptides from samples LHC and LHD were characterized. The results indicated that all eight isomers, containing three presumably native disulfide bonds and one free and one carboxymethylated sulfhydryl group, are formed on air oxidation of reduced partially carboxymethylated lysozyme. Since in each of these isomers the formation of one of the four native disulfide bonds is permanently blocked, it would follow that no one of the four disulfide bonds of native lysozyme is obligatory in the formation of the other three native disulfide bonds.
Keywords: protein folding, renaturation of reduced proteins, native hydrodynamic volume, intermediates, carboxymethylated lysozyme
Full text
PDF
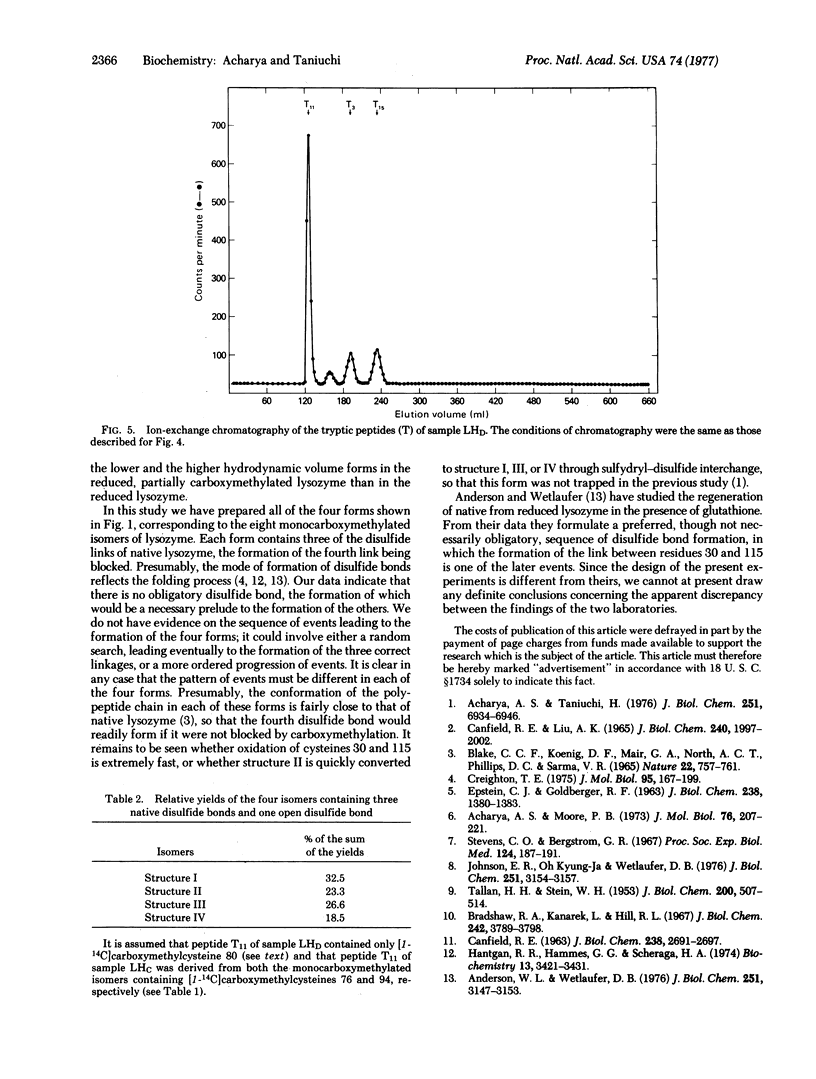
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B. Reaction of ribosomal sulfhydryl groups with 5,5'-dithiobis(2-nitrobenzoic acid). J Mol Biol. 1973 May 15;76(2):207–221. doi: 10.1016/0022-2836(73)90385-9. [DOI] [PubMed] [Google Scholar]
- Acharya A. S., Taniuchi H. A study of renaturation of reduced hen egg white lysozyme. Enzymically active intermediates formed during oxidation of the reduced protein. J Biol Chem. 1976 Nov 25;251(22):6934–6946. [PubMed] [Google Scholar]
- Anderson W. L., Wetlaufer D. B. The folding pathway of reduced lysozyme. J Biol Chem. 1976 May 25;251(10):3147–3153. [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Kanarek L., Hill R. L. The preparation, properties, and reactivation of the mixed disulfide derivative of egg white lysozyme and L-cystine. J Biol Chem. 1967 Sep 10;242(17):3789–3798. [PubMed] [Google Scholar]
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- CANFIELD R. E. PEPTIDES DERIVED FROM TRYPTIC DIGESTION OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2691–2697. [PubMed] [Google Scholar]
- Creighton T. E. The two-disulphide intermediates and the folding pathway of reduced pancreatic trypsin inhibitor. J Mol Biol. 1975 Jun 25;95(2):167–199. doi: 10.1016/0022-2836(75)90389-7. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Hammes G. G., Scheraga H. A. Pathways of folding of reduced bovine pancreatic ribonuclease. Biochemistry. 1974 Aug 13;13(17):3421–3431. doi: 10.1021/bi00714a001. [DOI] [PubMed] [Google Scholar]
- Johnson E. R., Oh K. J., Wetlaufer D. B. Formation of three-dimensional structure in protein fragments. Reactivation of reduced hen egg lysozyme fragment 1-127. J Biol Chem. 1976 May 25;251(10):3154–3157. [PubMed] [Google Scholar]
- Stevens C. O., Bergstrom G. R. The multiple nature of crystalline egg-white lysozyme. Proc Soc Exp Biol Med. 1967 Jan;124(1):187–191. doi: 10.3181/00379727-124-31697. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., STEIN W. H. Chromatographic studies on lysozyme. J Biol Chem. 1953 Feb;200(2):507–514. [PubMed] [Google Scholar]