Abstract
The vitellogenin gene is inactive in the liver of male Xenopus laevis, unless exogenous estrogen is administered. We have previously shown that conventional doses of estradiol-17beta result in the appearance of new hepatic messenger RNAs, some of which are encoded for vitellogenin. We now report that much higher doses of the hormone (2 mg/frog per day for 4 days) are required to elicit maximal responses. The relative levels of membrane-bound polysomes and vitellogenin mRNA were determined as a function of time and dose of hormone. Translation of total polysomal RNA in a cell-free system derived from wheat germ was used to estimate the relative levels of vitellogenin messenger RNA. Faithful translation of this messenger RNA was indicated by two lines of evidence: labeled cell-free products were immunoprecipitated with antivitellogenin antibody, and the migration of the major labeled product in sodium dodecyl sulfate/acrylamide gels was identical to that of native vitellogenin. Our results establish conditions for maximal estrogen-induced responses in this system, and are compatible with the hypothesis that a major regulatory mechanism of steroid hormones in the control of protein synthesis is that of gene activation and regulation of messenger RNA levels.
Full text
PDF
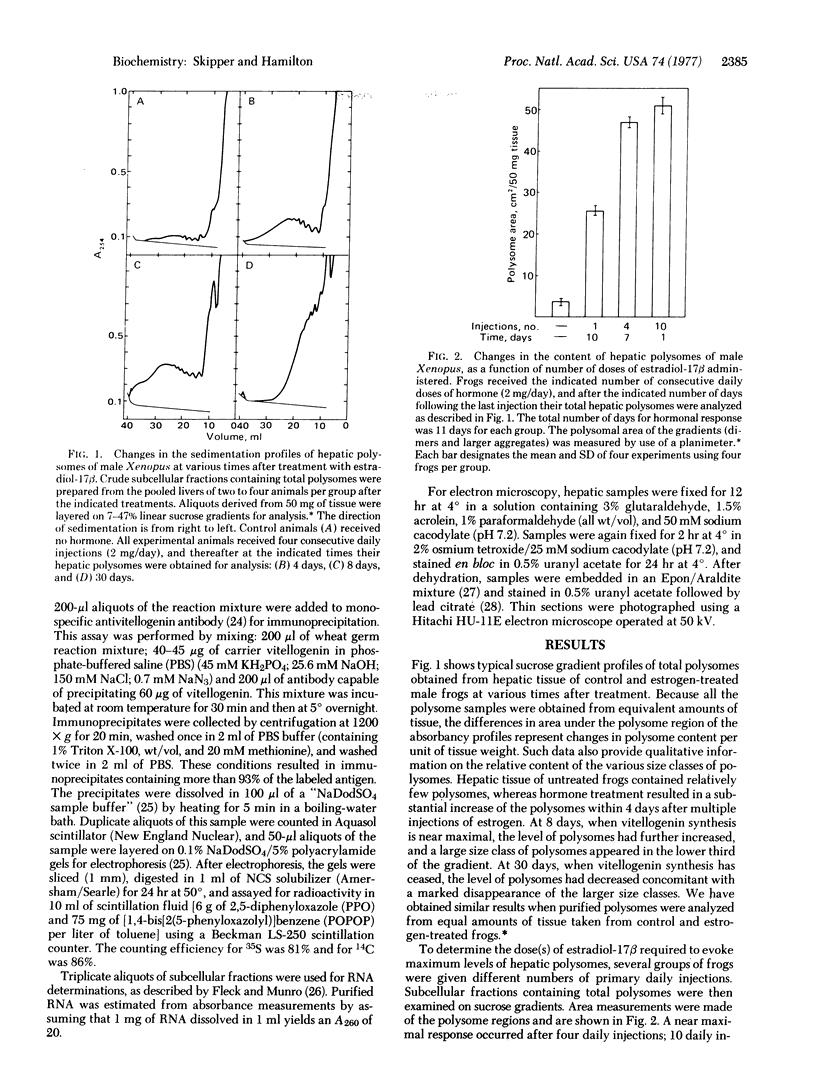
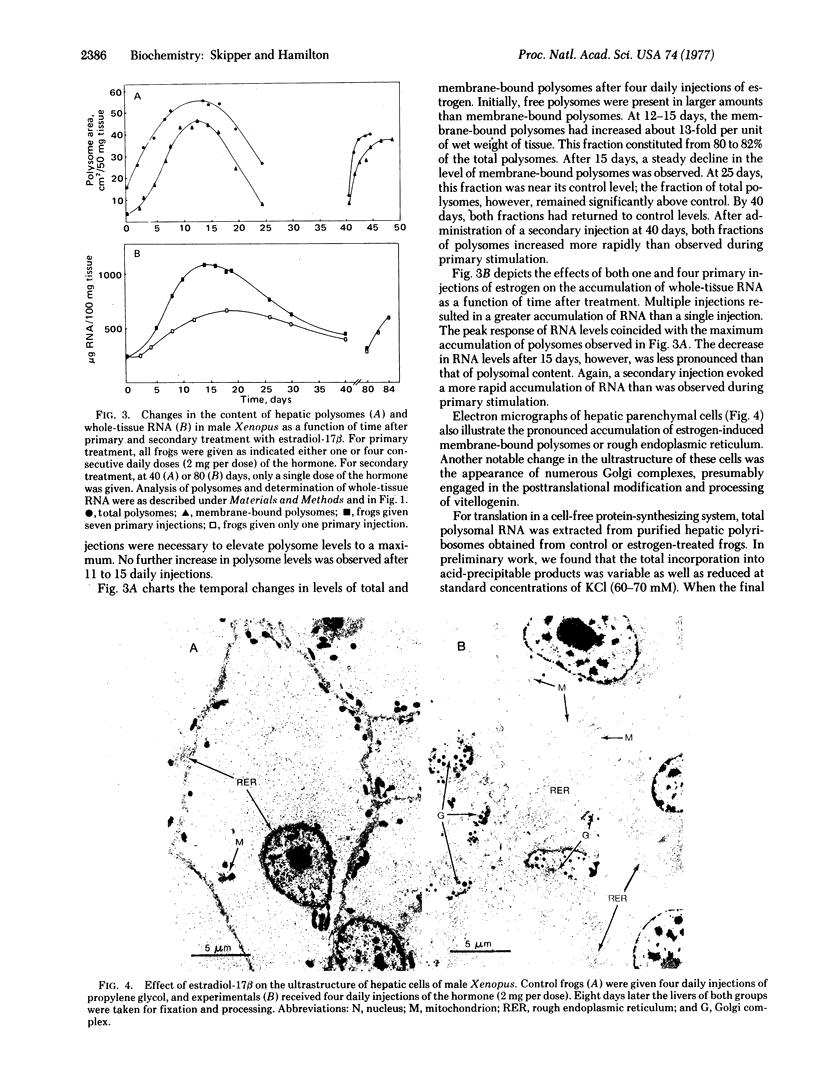
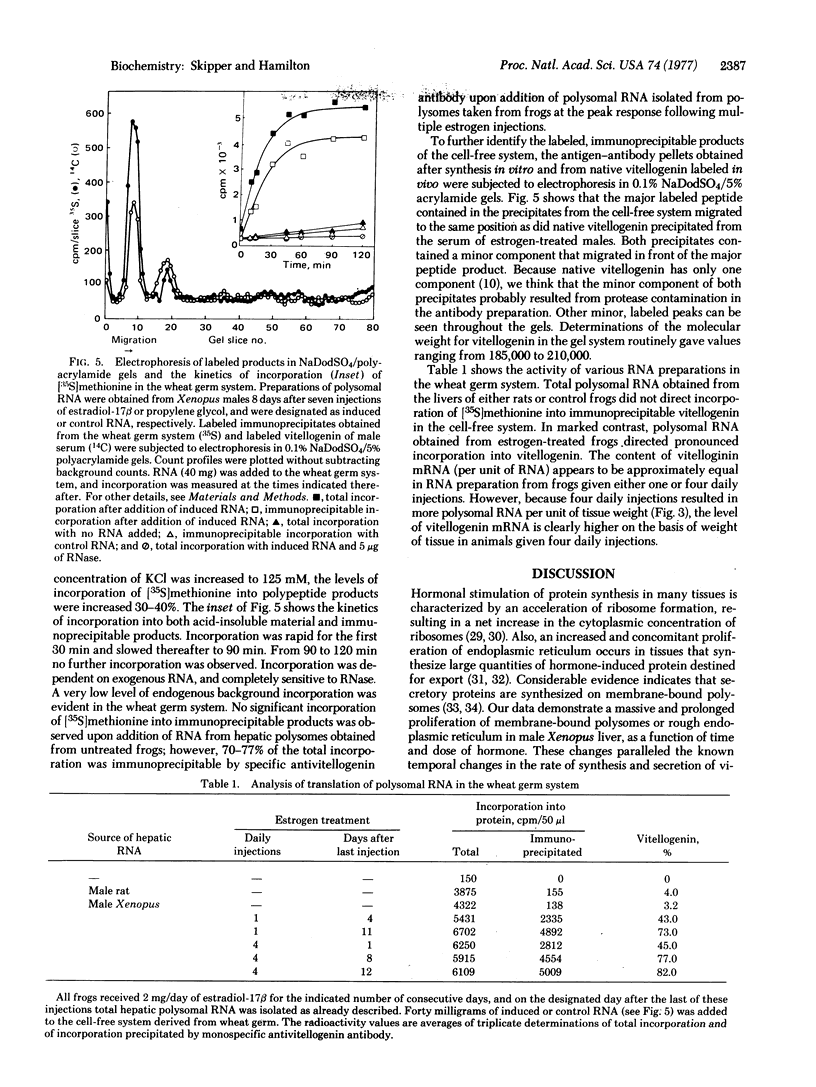

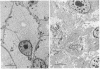
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink E. W., Wallace R. A. Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem. 1974 May 10;249(9):2897–2903. [PubMed] [Google Scholar]
- Berridge M. V., Farmer S. R., Green C. D., Henshaw E. C., Tata J. R. Characterization of polysomes from Xenopus liver synthesizing vitellogenin and translation of vitellogenin and albumin messenger RNA's in vitro. Eur J Biochem. 1976 Feb 2;62(1):161–171. doi: 10.1111/j.1432-1033.1976.tb10109.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Lane C. D. Translation of Xenopus liver messenger RNA in Xenopus oocytes: vitellogenin synthesis and conversion to yolk platelet proteins. Cell. 1976 Jun;8(2):283–297. doi: 10.1016/0092-8674(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Ribosomes in rat liver: an estimate of the percentage of free and membrane-bound ribosomes interacting with messenger RNA in vivo. J Mol Biol. 1967 Sep 28;28(3):539–542. doi: 10.1016/s0022-2836(67)80103-7. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Follett B. K., Redshaw M. R. The effects of oestrogen and gonadotrophins on lipid and protein metabolism in Xenopus laevis Daudin. J Endocrinol. 1968 Apr;40(4):439–456. doi: 10.1677/joe.0.0400439. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. The identification of polyribosomes synthesizing myosin. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1002–1009. doi: 10.1073/pnas.57.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanclos K. D., Hamilton T. H. Translation of hormone-induced messenger RNA in amphibian oocytes: I. Induction by estrogen of messenger RNA encoded for vitellogenic protein in the liver of the male African clawed toad (Xenopus laevis). Proc Natl Acad Sci U S A. 1975 Oct;72(10):3934–3938. doi: 10.1073/pnas.72.10.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- MOORE R. J., HAMILTON T. H. ESTROGEN-INDUCED FORMATION OF UTERINE RIBOSOMES. Proc Natl Acad Sci U S A. 1964 Aug;52:439–446. doi: 10.1073/pnas.52.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redshaw M. R., Follett B. K., Nichollis T. J. Comparative effects of the oestrogens and other steroid hormones on serum lipids and proteins in Xenopus laevis Daudin. J Endocrinol. 1969 Jan;43(1):47–53. doi: 10.1677/joe.0.0430047. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-1-C14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol. 1960 Jul;7:619–630. doi: 10.1083/jcb.7.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Mukherjee S. P. Isolation of messenger ribonucleic acid for myosin heavy chain. Prep Biochem. 1973;3(6):583–604. doi: 10.1080/00327487308061540. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Baker H. J., Stitt D. T. In vitro translation and estradiol-17beta induction of Xenopus laevis vitellogenin messenger RNA. J Biol Chem. 1976 May 25;251(10):3105–3111. [PubMed] [Google Scholar]
- Tata J. R. Co-ordination between membrane phospholipid synthesis and accelerated biosynthesis of cytoplasmic ribonucleic acid and protein. Biochem J. 1970 Feb;116(4):617–630. doi: 10.1042/bj1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N., Steele W. J. Free and membrane-bound polysomes of rat liver: separation in nearly quantitative yield and analysis of structure and function. Biochim Biophys Acta. 1972 Dec 22;287(3):526–537. doi: 10.1016/0005-2787(72)90298-5. [DOI] [PubMed] [Google Scholar]
- Wahli W., Wyler T., Weber R., Ryffel G. U. Size, complexity and abundance of a specific poly(A)-containing RNA of liver from male Xenopus induced to vitellogenin synthesis by estrogen. Eur J Biochem. 1976 Jul 15;66(3):457–465. doi: 10.1111/j.1432-1033.1976.tb10570.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. VII. Serum phosphoprotein synthesis by vitellogenic females and estrogen-treated males of Xenopus laevis. Can J Biochem. 1968 Aug;46(8):953–959. doi: 10.1139/o68-142. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Resolution and isolation of avian and amphibian yolk-granule proteins using TEAE-cellulose. Anal Biochem. 1965 May;11(2):297–311. doi: 10.1016/0003-2697(65)90018-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wittliff J. L., Kenney F. T. Regulation of yolk protein synthesis in amphibian liver. I. Induction of lipovitellin synthesis by estrogen. Biochim Biophys Acta. 1972 May 29;269(3):485–492. doi: 10.1016/0005-2787(72)90136-0. [DOI] [PubMed] [Google Scholar]