Abstract
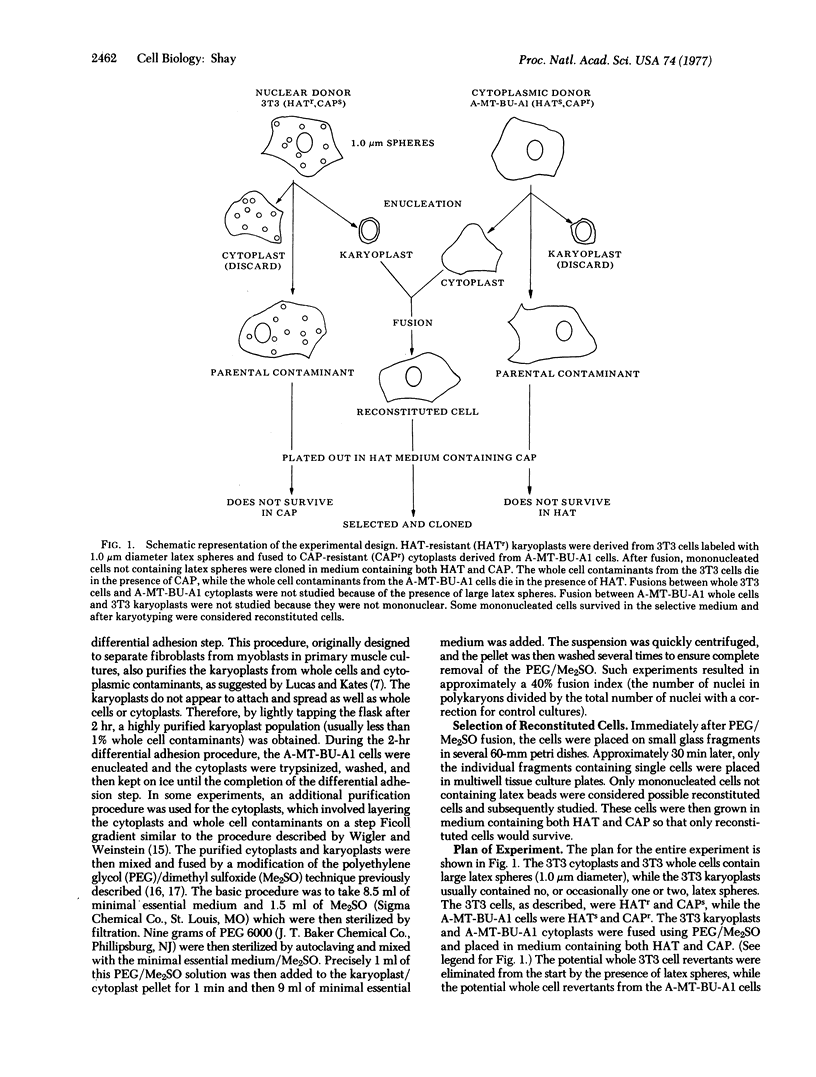
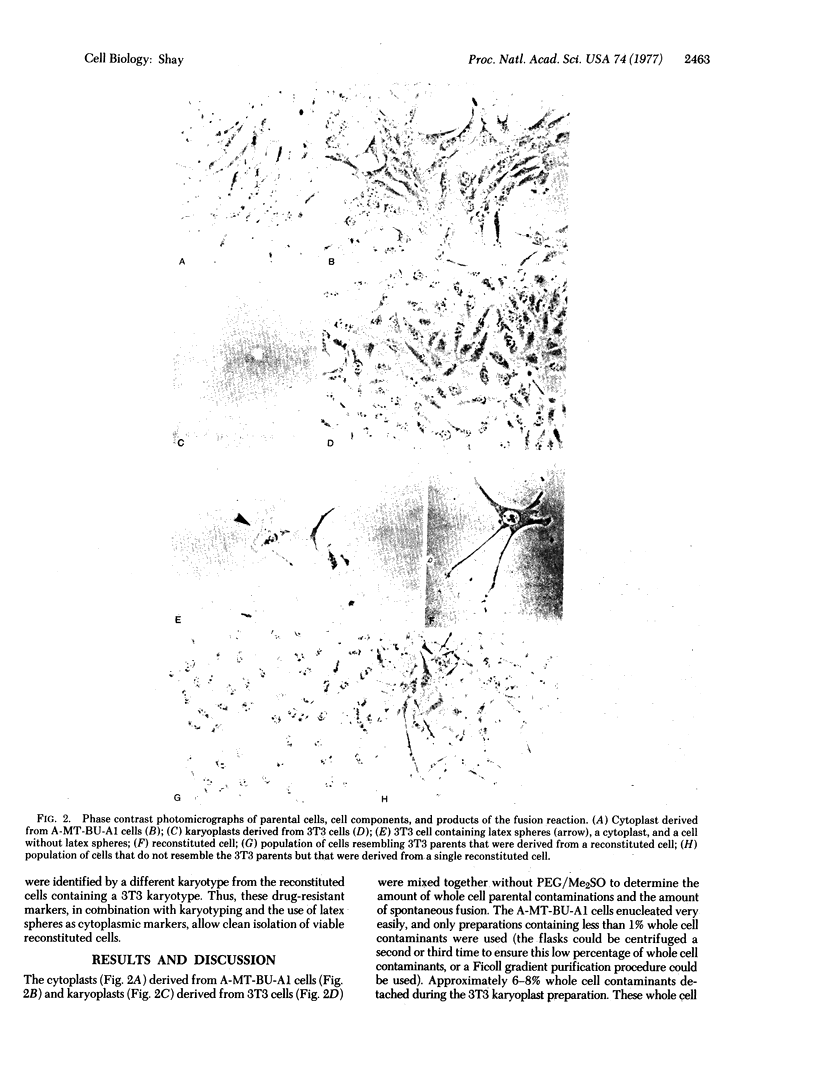
Murine Balb/3T3 and murine A-MT-BU-A1 mammary tumor cells were separated in the presence of cytochalasin B into enucleated cytoplasmic components (cytoplasts) and nucleated subcellular components (karyoplasts). Karyoplasts were derived from 3T3 cells, while cytoplasts were derived from A-MT-BU-A1 cells that were both chloramphenicol-resistant (CAPr) and sensitive to hypoxanthine/aminopterin/thymidine (HATs). CAPr has been shown to be cytoplasmically transmitted (possibly a mitochondrial gene mutation), while sensitivity to medium containing HAT has been shown to be transmitted by the nucleus (i.e., nuclear gene mutation). Such CAPr cytoplasts derived from A-MT-BU-A1 cells were then fused, using polyethylene glycol, to HAT-resistant 3T3 karyoplasts. The mononucleated reconstituted cells produced by such procedures were cloned in medium containing both HAT and CAP. Some of the reconstituted cells survived, because they were resistant to both drugs, while the nuclear and cytoplasmic whole cell contaminants were killed by one or the other of the two drugs. The results of these experiments indicate that reconstituted cells that are derived from two different cell lines are viable, as indicated by their ability for long-term proliferation in culture. Most of the clones derived resembled morphologically the 3T3 nuclear donor parent cells, but some of the clones did not resemble either parental cell line. It is anticipated that such selection techniques will permit more complete analysis of interrelationships between nucleus and cytoplasm.
Keywords: cytochalasin B, enucleation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Ege T., Hamberg H., Krondahl U., Ericsson J., Ringertz N. R. Characterization of minicells (nuclei) obtained by cytochalasin enucleation. Exp Cell Res. 1974 Aug;87(2):365–377. doi: 10.1016/0014-4827(74)90493-5. [DOI] [PubMed] [Google Scholar]
- Ege T., Krondahl U., Ringertz N. R. Introduction of nuclei and micronuclei into cells and enucleated cytoplasms by Sendai virus induced fusion. Exp Cell Res. 1974 Oct;88(2):428–432. doi: 10.1016/0014-4827(74)90267-5. [DOI] [PubMed] [Google Scholar]
- Ege T., Ringertz N. R. Viability of cells reconstituted by virus-induced fusion of minicells with anucleate cells. Exp Cell Res. 1975 Sep;94(2):469–473. doi: 10.1016/0014-4827(75)90520-0. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Kates J. R. The construction of viable nuclear-cytoplasmic hybrid cells by nuclear transplantation. Cell. 1976 Mar;7(3):397–405. doi: 10.1016/0092-8674(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Wivel N. A. Transfer of murine intracisternal A particle phenotype in chloramphenicol-resistant cytoplasts. Cell. 1976 Nov;9(3):383–391. doi: 10.1016/0092-8674(76)90083-0. [DOI] [PubMed] [Google Scholar]
- Muggleton-Harris A. L., Hayflick L. Cellular aging studied by the reconstruction of replicating cells from nuclei and cytoplasms isolated from normal human diploid cells. Exp Cell Res. 1976 Dec;103(2):321–330. doi: 10.1016/0014-4827(76)90269-x. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Zeigler C. J., Martin G. M. Dimethyl sulfoxide enhances polyethylene glycol-mediated somatic cell fusion. Somatic Cell Genet. 1976 May;2(3):263–270. doi: 10.1007/BF01538964. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Gershenbaum M. R., Porter K. R. Enucleation of CHO cells by means of cytochalasin B and centrifugation: the topography of enucleation. Exp Cell Res. 1975 Aug;94(1):47–55. doi: 10.1016/0014-4827(75)90529-7. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Porter K. R., Prescott D. M. The surface morphology and fine structure of CHO (Chinese hamster ovary) cells following enucleation. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3059–3063. doi: 10.1073/pnas.71.8.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veomett G., Prescott D. M., Shay J., Porter K. R. Reconstruction of mammalian cells from nuclear and cytoplasmic components separated by treatment with cytochalasin B. Proc Natl Acad Sci U S A. 1974 May;71(5):1999–2002. doi: 10.1073/pnas.71.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]