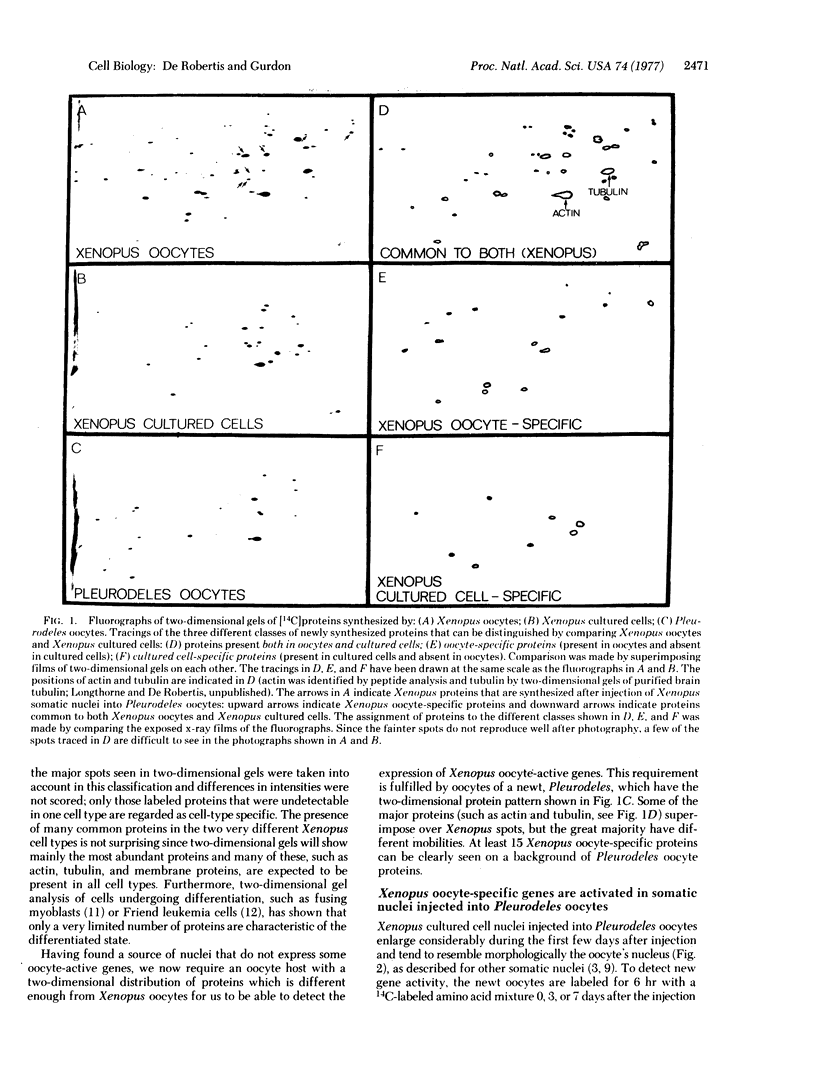
Abstract
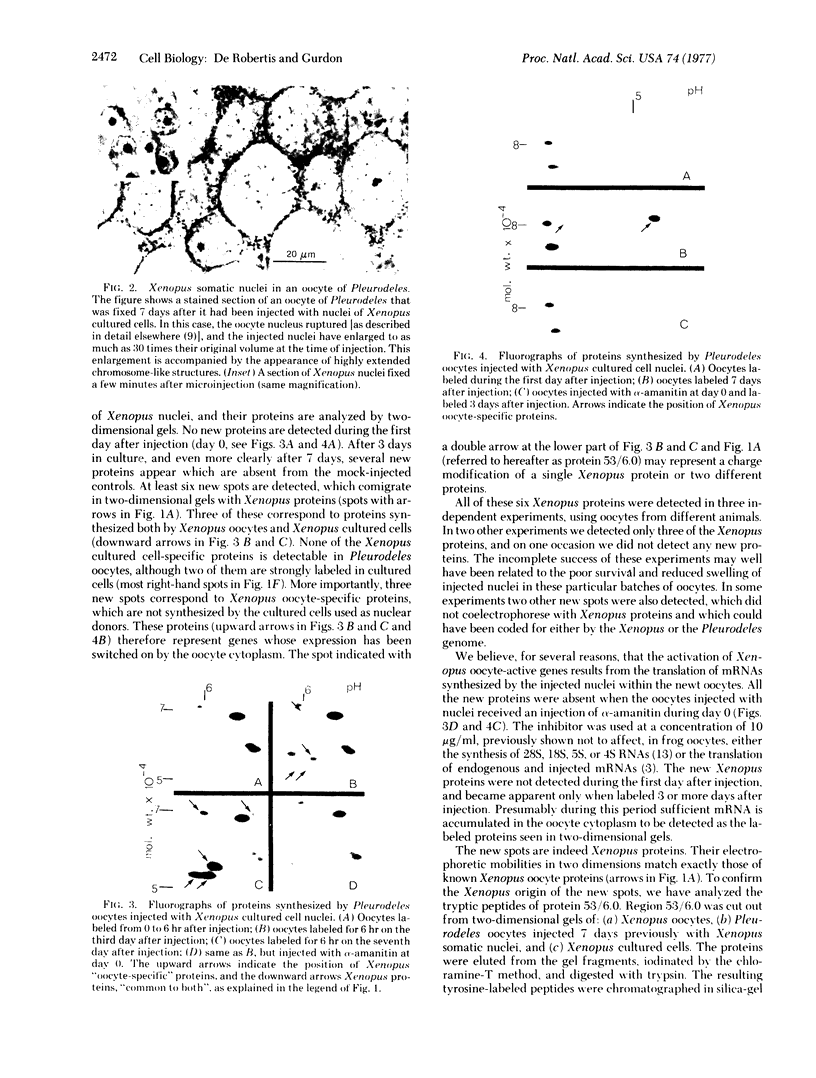
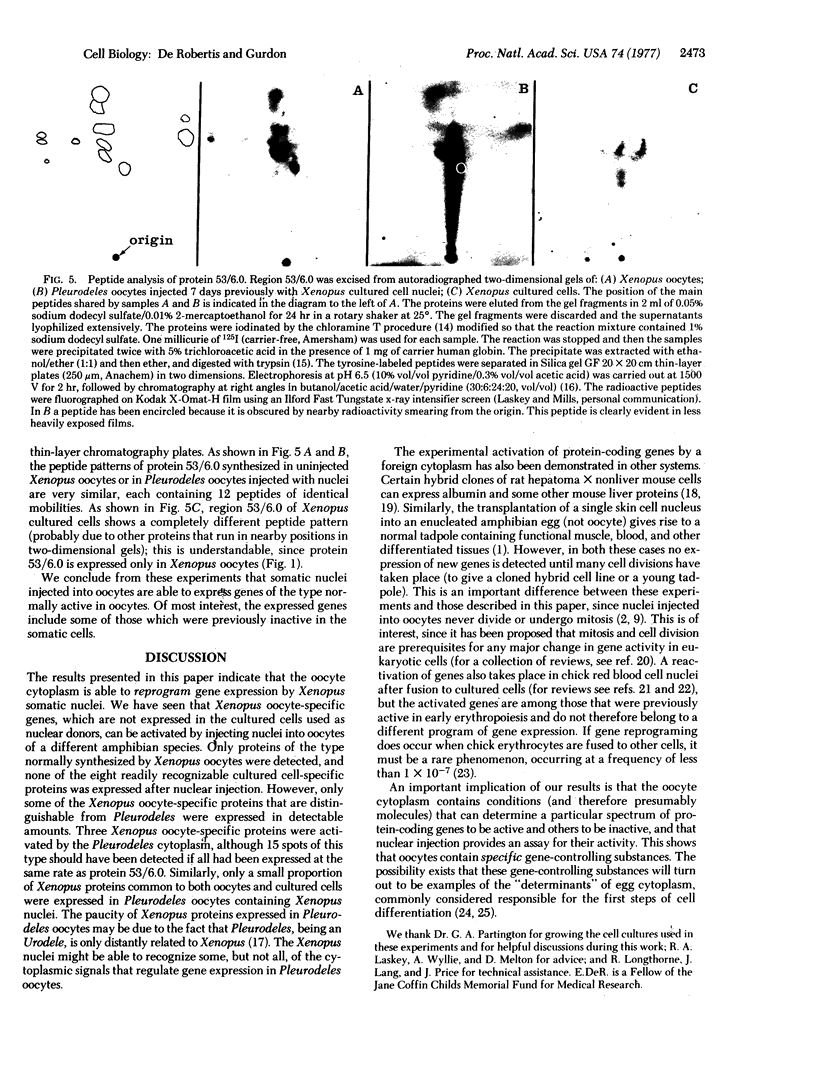
Genes that are unexpressed in somatic cells have been activated by injecting cultured cell nuclei of the frog Xenopus laevis into oocytes of the newt Pleurodeles waltlii. The genes that were activated are normally expressed in oocytes but not in cultured cells. Conversely, genes that are normally expressed in cultured cells but not in oocytes became inactive when cultured cell nuclei were injected into oocytes. These changes in gene activity were detected by two-dimensional gel electrophoresis of proteins synthesized by oocytes injected with nuclei. Controls, which included the use of alpha-amanitin, showed that these changes in protein synthesis are dependent on gene transcription. We conclude that genes that become inactive during cell differentiation can be reactivated, in the absence of cell division, by normal components of oocyte cytoplasm.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson S. A., Luger O., Ringertz N. R., Savage R. E. Phenotypic expression in chick erythrocyte x rat myoblast hybrids and in chick myoblast x rat myoblast hybrids. Exp Cell Res. 1974 Mar 15;84(1):47–55. doi: 10.1016/0014-4827(74)90378-4. [DOI] [PubMed] [Google Scholar]
- Colman A. Transcription of DNAs of known sequence after injection into eggs and oocytes of Xenopus laevis. Eur J Biochem. 1975 Sep 1;57(1):85–96. doi: 10.1111/j.1432-1033.1975.tb02279.x. [DOI] [PubMed] [Google Scholar]
- Etkin L. D. Regulation of lactate dehydrogenase (LDH) and alcohol dehydrogenase (ADH) synthesis in liver nuclei, following their transfer into oocytes. Dev Biol. 1976 Sep;52(2):201–209. doi: 10.1016/0012-1606(76)90240-2. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B., De Robertis E. M., Partington G. Injected nuclei in frog oocytes provide a living cell system for the study of transcriptional control. Nature. 1976 Mar 11;260(5547):116–120. doi: 10.1038/260116a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Gurdon J. B., Laskey R. A., Reeves O. R. The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. J Embryol Exp Morphol. 1975 Aug;34(1):93–112. [PubMed] [Google Scholar]
- Gurdon J. B., Partington G. A., De Robertis E. M. Injected nuclei in frog oocytes:RNA synthesis and protein exchange. J Embryol Exp Morphol. 1976 Dec;36(3):541–553. [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Sutasurya L. A. Embryological evidence for a possible polyphyletic origin of the recent amphibians. J Embryol Exp Morphol. 1976 Feb;35(1):159–167. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids: induction of mouse albumin production in rat hepatoma-mouse fibroblast hybrids. Proc Natl Acad Sci U S A. 1972 Mar;69(3):571–575. doi: 10.1073/pnas.69.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Proteins of Friend leukemia cells. Comparison of hemoglobin-synthesizing and noninduced populations. J Biol Chem. 1976 Jan 25;251(2):555–558. [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A., Mathews M. B. Translation of RNA from encephalomyocarditis virus in a mammalian cell-free system. Nature. 1970 Jan 10;225(5228):184–187. doi: 10.1038/225184a0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]