Abstract
Purpose
The aims of this study were to investigate the effects of manganese (Mn) dust exposure on lung functions and evaluate the potential synergistic effect between smoking and Mn dust exposure among refinery workers.
Methods
A retrospective study including 1658 workers in a ferromanganese refinery was conducted, with subjects who were from the Guangxi manganese-exposed workers healthy cohort (GXMEWHC). Based on the Mn manganese cumulative exposure index (Mn-CEI), all subjects were divided into the low exposure group (n = 682) and the high exposure group (n = 976). A pulmonary function test was performed using an electronic spirometer, including the values and percentages of FVC, FEV1, FEV1/FVC, MMEF, PEFR, MVV, respectively.
Results
No significant effect of Mn dust exposure on the pulmonary function was found in the female workers (all p>0.05). However, there was an obvious decrease in the male workers in the high exposure group compared with those in the low exposure group (FVC -60 ml, FEV1 -120 ml, MMEF -260 ml/s, MVV -5.06 L, all p<0.05). In the high exposure group, the reduction in FVC% predicted, MMEF and MMEF% predicted was 1.0%, 210 mL/s, and 4.9%, respectively. In particular, among the exposed subjects smokers had a statistically significant decrease in lung function compared with non-smokers and the reduction in FVC% predicted, MMEF and MMEF% predicted was 1.0%, 210 mL/s, and 4.9%, respectively (p<0.05). Partial correlation analysis showed that there was also negative correlation between Mn-CEI and decreased changes in MMEF (r = -0.159, p = 0.018) and also MMEF% predicted (r = -0.163, p = 0.015).
Conclusions
Mn dust can impair the pulmonary ventilation function of male workers but not females, and individual smoking habits and manganese exposure had a synergistic effect on the lung function decrease.
Introduction
Manganese (Mn) is an essential nutrient and it is necessary to maintain human health. In addition to food intake, environmental exposure is also the way to absorb Mn. The combustion products of methylcyclopentadienyl manganese tricarbonyl (MMT) in unleaded gasoline has been discharged approximately 30% Mn phosphate/sulfate mixture into the environment[1]. Similarly, a large number of occupational workers who work in industries such as welding, smelting, mining, ferroalloy and dry battery production are also exposed to high concentrations of Mn dust [2,3].
The major routes of Mn entry and absorption are the respiratory and gastrointestinal tracts[4], of which the proportion is 60% to 70% and 1% to 5% respectively [5].Therefore, the lung is one of the main target organs affected by long-term exposure to Mn dust [6]. Dorman et al. [7] reported that high-dose subchronic Mn sulfate inhalation was associated with increasing lung Mn concentrations. Previous studies also showed that long-term exposure to Mn dust led to workers’ lung function decrease in FVC, FEV1 and FEV1% pred [8,9]. However, the conclusions of these studies were inconsistent, and most of them were conducted without considering the long-term cumulative effect between manganese cumulative exposure index (Mn-CEI) and respiratory function impairment. Additionally, these included subjects were mostly male workers but not female workers. Therefore, based on our constructed Guangxi manganese-exposed workers healthy cohort (GXMEWHC) [10], we can further explore the effects of Mn dust exposure on the respiratory health in the cohort population.
Previous studies have shown that smoking is one of the most important identified causes of chronic obstructive pulmonary disease (COPD) and an aetiological factor for pancreatic cancer [11], and cigarette smoking is a key lifestyle factor that affects respiratory health [12,13]. In addition, smoking is considered to have a synergistic effect with tile dust exposure [14]. Langhammer et al. [15] found that smoking was associated with reduced lung function and increased prevalence of respiratory symptoms. In the GXMEWHC population, about 50% of male workers have the habit of smoking, but few studies to date have shown the potential interaction between smoking and Mn dust exposure.
Thus, the objectives of this study were undertaken to further substantiate early changes in the respiratory system in the GXMEWHC with long-term exposure to Mn dust and to evaluate the potential synergistic effect between Mn dust and smoking.
Materials and Methods
Study population
We established the GXMEWHC which consists of 1888 workers in the ferromanganese refinery from July 2011 to September 2012, as previously described [16], 1658 workers were finally recruited according to inclusion criteria. The main criteria for participation were employment for at least 3 months, no diabetes mellitus or serious kidney or liver diseases, lung diseases that are probably related to Mn exposure, and no physical disability or surgical history. Additionally, the exclusion criteria included chronic obstructive pulmonary diseases and unwillingness to participate in the interview.
The study was approved by the ethics and human subjects committee of Guangxi Medical University. All participants signed informed consent forms before the start of this study.
Questionnaire
Basic demographic information was collected using a specifically designed questionnaire after obtaining written informed consent. The questionnaire also included drinking status, smoking status, disease history, medication and occupational history, and socio-economic factors. Drinking status was defined as current drinking (drinking at least once each week for more than 3 months), former drinking (the person had stopped drinking for at least 3 months) and never drinking. Smoking status was defined as current smoking (smoking at least one cigarette daily for more than 3 months), former smoking (stopped smoking for at least 3 months) and never smoking. We calculated the smoking index from the number of cigarettes smoked per day and multiplied it by years of smoking.
Pulmonary function tests
The pulmonary function was recorded by a spirometer (SiChuan sikeda S-980A Technology Co., Ltd. China) according to the standards of the American Thoracic Society[17]. We measured the values and percentages of forced vital capacity (FVC), forced expiratory volume at one second (FEV1), the ratio of forced expiratory volume at one second (FEV1%), maximal med-expiratory flow curve (MMEF), peak expiratory flow ratio (PEFR), and maximal voluntary ventilation (MVV).
FEV1 (L) and FVC (L) were measured with the best value of three forced expiratory manoeuvres in the standing position. The largest and second-largest FVC measurements should be within 100 ml or 0.5% of each other when expressed as a percentage of the larger FVC. The FVC and FEV1 values in per cent of predicted (% pred) were calculated according to the ECCS reference values [18]. The mean per cent predicted value was based on subject age, height, weight, sex and ethnic group as calculated and adjusted by the spirometer device. The percentage predicted lung values were observed capacities as measured by a spirometer divided by predicted or expected capacities multiple by 100 [19].
All respiratory function tests were carried out at a fixed time of the day (8.00 am to 12.00 pm) to minimize the diurnal variation [20].
Manganese exposure assessment
We had recorded the technological processes of production and the situation of potential Mn pollution in this company. We detected the individual levels of Mn by individual samplers during on-job time. Air samples were collected by FC-2 dust samplers according to the standard specification issued by the Ministry of Health in China-Specifications of air sampling for hazardous substances monitoring in the workplace (GBZ 159–2004), and the method had been described in detail in previous studies [21]. Permissible concentration-time-weighted average (PC-TWA) is the average permissible exposure levels on the regulation eight hours working day weighting by time. The cumulative exposure index (CEI) is calculated which combines PC-TWA and the working seniority. As Mn external exposure index, the Mn-CEI was calculated for each subject referred to the individual airborne monitoring in the working time and also breaking time [10].
We divided all subjects into two groups based on the Mn-CEI [22] including the low exposure group (Mn-CEI<1.00 mg/m3.year) and the high exposure group (Mn-CEI≥1.00 mg/m3.year).
Statistical analysis
All analyses were performed by the SPSS statistical program, version 16.0. Analysis of covariance was used to compare continuous variables between groups by estimating the mean ± SE. The discrete variables were evaluated using a Chi-Square test. We used analysis of covariance (ANOVA) to compare the differences of lung functions between the high exposure group and the low exposure group, adjusted by the level of age, height, weight and smoking index. Partial correlation analysis was applied for the relation between Mn-CEI and pulmonary function values of workers, adjusted by the above confounding factors. All statistical tests were two-tailed and the level of significance was achieved at p < 0.05.
Results
Manganese concentration in the workplaces
The high exposure workplaces mainly included smelters, human crushing worker and craneman, and the Mn concentration in these workplaces ranged from 0.015 to 0.363 mg/m3. Additionally, the low exposure workplaces were mainly comprised auxiliary workers, such as car driver, alkali recovery worker and electrician, and the Mn concentrations in these workplaces ranged from 0.004 to 0.140 mg/m3. The Mn-CEI was in the range 0.010–10.300 mg/m3.year and median was 1.290 mg/m3. year in all workers. The details of the PC-TWA and the Mn-CEI results are shown in S1 Table and S2 Table.
Characteristics of the study population
As presented in Table 1, a total of 1658 subjects enter this study, including low exposure group (n = 682) and high exposure group (n = 976). The low exposure group comprised 407 men (59.7%) and 275 women (40.3%), and the high exposure group comprised 632 men (64.8%) and 345 women (35.3%). The average age was 37.6 years for the low exposure workers and 42.6 years for the high exposure workers. Of the low exposure workers, 348 (51.0%) were current smokers and 350 (51.3%) current drinkers. Of the high exposure workers, 289 (29.6%) were current smokers and 431 (44.1%) current drinkers. Significant difference was found between the low exposure group and the high exposure group regarding the mean age, seniority, smoking distribution, drinking distribution and gender, p<0.05, respectively.
Table 1. Demographic data of the study population.
| Low exposure group | High exposure group | ||
|---|---|---|---|
| 0>Mn-CEI<1 (N = 682) | Mn-CEI≥1 (N = 976) | ||
| Variables | Mean ± SD | Mean ± SD | P value |
| Age(years) | 37.58±8.40 | 42.57±6.35 | 0.000 |
| Seniority(years) | 11.46±9.62 | 18.55±8.48 | 0.000 |
| Height(cm) | 163.27±7.64 | 162.93±7.35 | 0.361 |
| Weight(kg) | 59.71±9.41 | 60.26±9.57 | 0.248 |
| Sex | |||
| Male | 407(59.7) | 631(64.7) | 0.039 |
| Female | 275(40.3) | 345(35.3) | |
| Smoking status | |||
| Current smoker | 348(51.0) | 289(29.6) | 0.000 |
| Former smoker | 70(10.3) | 52(5.3) | |
| Never smoke | 264(38.7) | 635(65.1) | |
| Drinking status | |||
| Current drinker | 350(51.3) | 431(44.1) | 0.002 |
| Former drinker | 113(16.6) | 151(15.5) | |
| Never drink | 219(32.1) | 394(40.4) |
SD, standard deviation
Effects of the Mn-CEI on lung function outcomes among males
Table 2 describes the effects of different variables on lung function, adjusted by the levels of age, height, weight and smoking index. Average FEV1, FEV1% pred, FEV1/FVC, FEV1/FVC% pred, MMEF, MMEF% pred, MVV and MVV% pred was lower in the high exposure workers than those in the the low exposure group (all p<0.05). Compared with the low exposure group, the adjusted impaired effects were-60 ml on FVC, -120 ml on FEV1, -260 ml/s on MMEF and 5.06 L on MVV in the high exposure group. There was a statistically significant difference between the high exposure group and the low exposure group in FEV1, FEV1/FVC, MMEF and MVV (p<0.05).
Table 2. Adjusted effects on lung function of males by the levels of age, height, weight and smoking index.
| Low exposure group | High exposure group | |||||
|---|---|---|---|---|---|---|
| 0>Mn-CEI<1 (N = 407) | Mn-CEI≥1 (N = 631) | |||||
| Variables | Mean | SE | Mean | SE | P value | |
| FVC, L | 4.26 | 0.04 | 4.20 | 0.03 | 0.223 | |
| FVC% pred | 110.15 | 0.91 | 108.44 | 0.72 | 0.150 | |
| FEV1, L | 3.67 | 0.03 | 3.55 | 0.30 | 0.003 | |
| FEV1% pred | 112.82 | 0.99 | 109.96 | 0.78 | 0.003 | |
| FEV1/FVC | 86.52 | 0.50 | 85.01 | 0.39 | 0.021 | |
| FEV1/FVC% pred | 112.54 | 0.66 | 110.86 | 0.52 | 0.051 | |
| MMEF,L/s | 4.67 | 0.07 | 4.41 | 0.05 | 0.003 | |
| MMEF% pred | 104.92 | 1.48 | 98.95 | 1.18 | 0.002 | |
| PEFR,L/min | 8.20 | 0.13 | 8.17 | 0.10 | 0.873 | |
| PEFR% pred | 90.85 | 1.42 | 88.80 | 1.12 | 0.272 | |
| MVV,L | 146.78 | 1.27 | 141.72 | 1.01 | 0.003 | |
| MVV% pred | 147.01 | 1.30 | 142.44 | 1.03 | 0.011 | |
The results of the analysis of covariance are shown adjusted for age (y) and height (cm), weight (kg) and smoking index. SE, standard error.
Effects of the Mn-CEI on lung function outcomes among females
As shown in Table 3, the effects of different variables on lung function are adjusted by the level of age, height and weight. Average FVC, FVC% pred, FEV1, FEV1% pred, FEV1/FVC, MMEF, MMEF% pred, MVV and MVV% pred was higher in the high exposure workers than the low exposure group except FEV1/FVC% pred, but there was no statistically significant difference between the high exposure group and the low exposure group, all p >0.05.
Table 3. Adjusted effects on lung function of female workers by the levels of age, height and weight.
| Low exposure group | High exposure group | ||||
|---|---|---|---|---|---|
| 0<Mn-CEI<1 (N = 275) | Mn-CEI≥1 (N = 345) | ||||
| Variables | Mean | SE | Mean | SE | P value |
| FVC, L | 3.11 | 0.03 | 3.16 | 0.03 | 0.298 |
| FVC% pred | 110.84 | 1.11 | 113.62 | 0.99 | 0.073 |
| FEV1, L | 2.67 | 0.03 | 2.70 | 0.03 | 0.412 |
| FEV1% pred | 110.76 | 1.20 | 113.53 | 1.07 | 0.087 |
| FEV1/FVC | 86.15 | 0.56 | 86.25 | 0.49 | 0.893 |
| FEV1/FVC% pred | 105.98 | 0.68 | 105.91 | 0.61 | 0.946 |
| MMEF,L/s | 3.39 | 0.06 | 3.40 | 0.05 | 0.860 |
| MMEF% pred | 98.54 | 1.63 | 100.17 | 1.45 | 0.466 |
| PEFR,L/min | 5.43 | 0.10 | 5.48 | 0.09 | 0.695 |
| PEFR% pred | 82.53 | 1.55 | 84.64 | 1.38 | 0.322 |
| MVV,L | 106.98 | 1.16 | 108.14 | 1.03 | 0.467 |
| MVV% pred | 127.15 | 1.35 | 129.58 | 1.20 | 0.191 |
The results of the analysis of covariance are shown adjusted for age (y) and height (cm), weight (kg). SE, standard error.
Synergism between the Mn dust and smoking
Table 4 shows the results of potential synergism between Mn cumulative exposure (Mn-CEI) and smoking among male subjects. Confounding factors were adjusted including age, height, and weight. Compared with non-smokers, smokers have lower lung function values in both the low exposure group and the high exposure group. The averages of all lung parameters of the high exposure group were lower than the low exposure group. In the high exposure group, we also found that the adverse effects of manganese dust exposure on lung function were larger in smokers than in non-smokers. The reduction in FVC% pred, MMEF and MMEF% pred was 1.0%, 210 mL/s, and 4.9%, respectively. A similar pattern was found for other lung function parameters, although the reductions in lung function parameters were not statistically significant (p>0.05).
Table 4. Pulmonary function values for males by smoking habit per study stage.
| Low exposure group | High exposure group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0<Mn-CEI<1 (N = 407) | Mn-CEI≥1 (N = 631) | |||||||||||
| non-smoking (n = 113) | Smoking (n = 294) | non-smoking (n = 405) | Smoking (n = 226) | |||||||||
| Variables | Mean | SE | Mean | SE | P value | Mean | SE | Mean | SE | P value | ||
| FVC, L | 4.40 | 0.06 | 4.39 | 0.04 | 0.889 | 4.19 | 0.05 | 4.09 | 0.04 | 0.094 | ||
| FVC% pred | 11.08 | 1.60 | 111.01 | 0.99 | 0.969 | 109.74 | 1.19 | 106.79 | 0.89 | 0.047 | ||
| FEV1, L | 3.79 | 0.06 | 3.79 | 0.04 | 0.918 | 3.51 | 0.04 | 3.46 | 0.03 | 0.377 | ||
| FEV1% pred | 113.30 | 1.81 | 113.58 | 1.12 | 0.896 | 110.03 | 1.28 | 107.62 | 0.95 | 0.132 | ||
| FEV1/FVC | 86.23 | 0.87 | 86.97 | 0.54 | 0.475 | 85.20 | 0.49 | 84.2 | 0.66 | 0.225 | ||
| FEV1/FVC% pred | 110.60 | 1.16 | 111.21 | 0.72 | 0.657 | 112.39 | 0.65 | 111.01 | 0.87 | 0.204 | ||
| MMEF,L/s | 4.71 | 0.12 | 4.85 | 0.07 | 0.307 | 4.46 | 0.09 | 4.25 | 0.06 | 0.052 | ||
| MMEF% pred | 101.57 | 2.60 | 105.08 | 1.61 | 0.253 | 102.66 | 1.96 | 97.73 | 1.47 | 0.045 | ||
| PEFR,L/min | 8.49 | 0.25 | 8.40 | 0.15 | 0.751 | 8.22 | 0.16 | 7.97 | 0.12 | 0.218 | ||
| PEFR% pred | 92.67 | 2.67 | 91.66 | 1.65 | 0.746 | 90.13 | 1.81 | 87.07 | 1.34 | 0.175 | ||
| MVV,L | 151.34 | 2.36 | 151.74 | 1.46 | 0.886 | 140.32 | 1.62 | 138.32 | 1.21 | 0.325 | ||
| MVV% pred | 149.17 | 2.36 | 147.62 | 1.46 | 0.576 | 144.06 | 1.70 | 140.58 | 1.27 | 0.101 | ||
Partial correlation analysis between Mn-CEI and pulmonary function values
As presented in Table 5, in high exposure group, there was significant negative correlation between cumulative exposure to manganese and changes in MMEF, MMEF% pred (p<0.05). A similar pattern was found for other lung function parameters, although the reductions in lung function parameters were not statistically significant, p>0.05.
Table 5. Partial correlation analysis between Mn-CEI and pulmonary function values of workers.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Variables | r1 (N = 226) | P value | r2 (N = 405) | P value | r3 (N = 618) | P value |
| FVC, L | -0.072 | 0.287 | -0.056 | 0.260 | 0.029 | 0.468 |
| FVC% pred | -0.073 | 0.280 | -0.021 | 0.677 | 0.050 | 0.216 |
| FEV1, L | -0.087 | 0.198 | -0.092 | 0.064 | 0.066 | 0.104 |
| FEV1% pred | -0.107 | 0.111 | -0.031 | 0.534 | 0.071 | 0.079 |
| FEV1/FVC | -0.027 | 0.686 | -0.067 | 0.177 | 0.039 | 0.332 |
| FEV1/FVC% pred | -0.042 | 0.533 | -0.037 | 0.456 | 0.035 | 0.392 |
| MMEF,L/s | -0.159 | 0.018 | -0.055 | 0.268 | 0.042 | 0.293 |
| MMEF% pred | -0.163 | 0.015 | -0.021 | 0.674 | 0.051 | 0.205 |
| PEFR,L/min | -0.058 | 0.392 | -0.040 | 0.420 | 0.074 | 0.067 |
| PEFR% pred | -0.086 | 0.200 | -0.040 | 0.424 | 0.086 | 0.079 |
| MVV,L | -0.087 | 0.197 | -0.089 | 0.074 | 0.066 | 0.103 |
| MVV% pred | -0.043 | 0.525 | -0.032 | 0.524 | 0.070 | 0.615 |
r1, the partial correlation coefficients of smoking male workers in the high exposure group
r2, the partial correlation coefficients of non-smoking male workers in the high exposure group
r3, the partial correlation coefficients of female workers
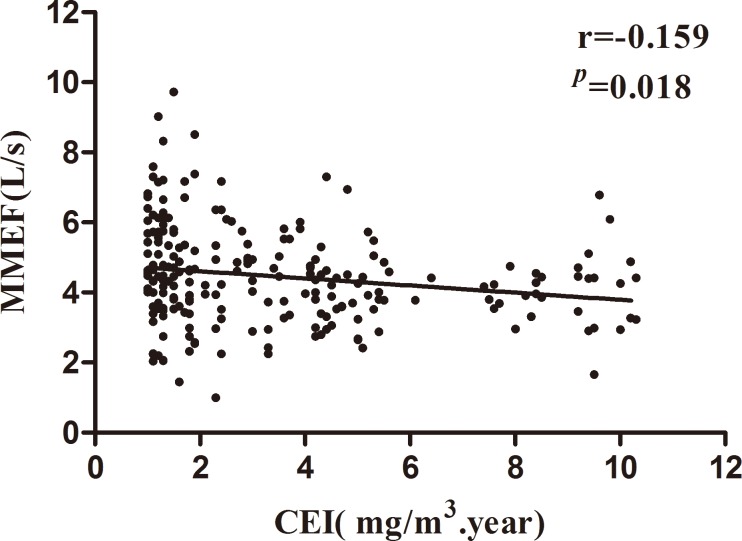
In the correlation analysis, a decreased linear trend of MMEF was found in the exposed subjects with the increased dose of Mn-CEI in the scatter diagram, and a significant negative correlation between MMEF and CEI (r = -0.159, p<0.05) was shown among smoking workers in high exposure group, as shown in Fig. 1.
Figure 1. Correlation of maximal med-expiratory flow curve (MMEF) and the manganese cumulative exposure index (Mn-CEI) in male workers among high exposure group.
Data were analyzed by partial correlation, r = -0.159, p<0.01.
Discussion
In order to explore potential adverse effects of manganese dust exposure on lung function in ferromanganese works, we investigated 1658 workers who were employed in a ferromanganese refinery in Guangxi. We also addressed potential interaction between smoking and manganese dust exposure, whether smoking would modify the impact of manganese dust exposure on lung functions.
Soyseth et al. and Antonini et al. found that manganese dust exposure adversely affects pulmonary function including airflow limitation, bronchitis, airway irritation, lung function changes [23,24]. Our data also found that ferroalloy working was related to reduce lung ventilation function. Our main finding was that long-term Mn dust exposure for male workers was related to a significant decrease in FEV1, FEV1% pred, MMEF and MMEF% pred, FEV1/FVC, MVV and MVV% pred. FEV1 is the main indicator of pulmonary function damage and FEV1/FVC is a parameter of obstructive pulmonary dysfunction, These results might indicate potential impairment of medium and large airways, and an increase in the prevalence of obstructive ventilator disorder among male workers [25]. Loukzadeh et al. [26] and Bowler et al. [22] also found that the average of FEV1 and FEV1/FVC were significantly lower in high exposure welders than low exposure welders. However, Johnsen [27] found that only FEV1 annual declined among employees in the smelting industry; the main reason may be differences in lung function measurement methods and basic characteristics of the study population. In addition, we did not find Mn dust exposure adversely affected pulmonary function among female workers. This may be due to women generally being assigned to tasks that had lower manganese exposure [28] and possible stronger healthy worker effects among women [29]. In this cohort population, we explored the potential synergistic effect between smoking and Mn dust exposure but the results showed that lung function was only statistically significant reduced in FVC% pred, MMEF and MMEF% pred in the higher exposure group. This is in consistent with findings of studies accomplished by Boojar et al.[4] and Beckett et al. [30] which effects was only seen in exposed workers compared with controls. Like our study, Sharifian et al. found no significant decreased pulmonary function parameters (FEV1, FEV1/FVC, PEFR) and smoking [31]. The lung function of smoking and non-smoking workers was similar in the low exposure group. These results, however, might be interpreted with caution because the low exposure group population was younger than 40 years, and exposure duration was probably not long enough for airflow limitation to become apparent [32]. Nicola et al. found that smoking history was not associated with pulmonary function decline in young smokers, which might be due to the pulmonary function tests being unable to detect early physiologic changes in the airways [33].
The MMEF is particularly reduced when the small airways function is impaired, as well as parenchymal damage [34]. In the correlation analysis, a significant correlation between MMEF, MMEF% pred and Mn-CEI was shown among smoking workers in the high exposure group. These results might indicate the function of small airways was injured due to an increase in mucosal secretions and perhaps decrease in the diameter of respiratory ducts. Similarly, Nemoto et al. [35] epidemiologically demonstrated the harmful effect of long-term cigarette smoke inhalation on MEFs in male smokers. Dorman et al. [7] also reported that high-dose subchronic manganese sulfate inhalation was associated with small airway inflammatory changes in the absence of observable clinical signs. In addition, this study did not find a negative correlation between cumulative exposure to Mn and FVC, FEV1, but the lung function of male workers has changed. Therefore, we need to continually follow up this aspect among regarding workers.
Our study had some limitations. First, this survey was designed as a retrospective study, therefore, further prospective studies are needed to better clarify the nature of the observed association and even other lung diseases such as asthma and COPD. Second, we only evaluated the effects of manganese dust on lung function of workers, and we did not collect other potential harmful factors which affected lung function, such as harmful gas including carbon monoxide and nitrogen oxide.
Conclusions
This study showed that the risk of decreasing lung function was associated with occupational cumulative exposure to manganese dust in male smelter workers, and individual smoking habits and manganese exposure had a synergistic effect on the lung function decrease. No significant relationship was found between female workers and manganese dust exposure. In order to investigate the relationship between occupational exposure and impairment of lung function, longitudinal studies are required in our cohort.
Supporting Information
(DOC)
(DOC)
Acknowledgments
We thank all the participants who volunteered to take part in this study, all members of the GXMEWHC cohort research team, the nurses and administrators in the ferromanganese refinery.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by National Natural Science Foundation of China (81472962, 21167004, and 81160339); Guangxi Science Fund for Distinguished Young Scholars (2012jjFA40011); Guangxi Natural Science Foundation (2011jjA40294); Guangxi science and technology development project (1355007-1); and Program for New Century Excellent Talents in University (NCET-12-0653). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beaupre LA, Salehi F, Zayed J, Plamondon P, L'Esperance G (2004) Physical and chemical characterization of mn phosphate/sulfate mixture used in an inhalation toxicology study. Inhal Toxicol 16: 231–244. [DOI] [PubMed] [Google Scholar]
- 2. Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, et al. (2003) The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology 24: 885–894. [DOI] [PubMed] [Google Scholar]
- 3. Levy BS, Nassetta WJ (2003) Neurologic effects of manganese in humans: a review. Int J Occup Environ Health 9: 153–163. [DOI] [PubMed] [Google Scholar]
- 4. Boojar MM, Goodarzi F (2002) A longitudinal follow-up of pulmonary function and respiratory symptoms in workers exposed to manganese. J Occup Environ Med 44: 282–290. [DOI] [PubMed] [Google Scholar]
- 5. Burton NC, Guilarte TR (2009) Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect 117: 325–332. 10.1289/ehp.0800035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorman DC, Struve MF, Norris A, Higgins AJ (2008) Metabolomic analyses of body fluids after subchronic manganese inhalation in rhesus monkeys. Toxicol Sci 106: 46–54. 10.1093/toxsci/kfn159 [DOI] [PubMed] [Google Scholar]
- 7. Dorman DC, Struve MF, Gross EA, Wong BA, Howroyd PC (2005) Sub-chronic inhalation of high concentrations of manganese sulfate induces lower airway pathology in rhesus monkeys. Respir Res 6: 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wittczak T, Dudek W, Krakowiak A, Walusiak J, Palczynski C (2008) Occupational asthma due to manganese exposure: a case report. Int J Occup Med Environ Health 21: 81–83. 10.2478/v10001-007-0041-1 [DOI] [PubMed] [Google Scholar]
- 9. Johnsen HL, Kongerud J, Hetland SM, Benth JS, Soyseth V (2008) Decreased lung function among employees at Norwegian smelters. Am J Ind Med 51: 296–306. 10.1002/ajim.20557 [DOI] [PubMed] [Google Scholar]
- 10. Lv Y, Zou Y, Liu J, Chen K, Huang D, et al. (2014) Rationale, design and baseline results of the Guangxi manganese-exposed workers healthy cohort (GXMEWHC) study. BMJ Open 4: e005070 10.1136/bmjopen-2014-005070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zou L, Zhong R, Shen N, Chen W, Zhu B, et al. (2014) Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer 50: 193–203. 10.1016/j.ejca.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 12. Steliga MA, Dresler CM (2011) Epidemiology of lung cancer: smoking, secondhand smoke, and genetics. Surg Oncol Clin N Am 20: 605–618. 10.1016/j.soc.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 13. Melville AM, Pless-Mulloli T, Afolabi OA, Stenton SC (2010) COPD prevalence and its association with occupational exposures in a general population. Eur Respir J 36: 488–493. 10.1183/09031936.00038309 [DOI] [PubMed] [Google Scholar]
- 14. Jaakkola MS, Sripaiboonkij P, Jaakkola JJ (2011) Effects of occupational exposures and smoking on lung function in tile factory workers. Int Arch Occup Environ Health 84: 151–158. 10.1007/s00420-010-0603-6 [DOI] [PubMed] [Google Scholar]
- 15. Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L (2003) Sex differences in lung vulnerability to tobacco smoking. Eur Respir J 21: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 16. Deng Q, Liu J, Li Q, Chen K, Liu Z, et al. (2013) Interaction of occupational manganese exposure and alcohol drinking aggravates the increase of liver enzyme concentrations from a cross-sectional study in China. Environ Health 12: 30 10.1186/1476-069X-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(1995) Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 18. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, et al. (1993) Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16: 5–40. [PubMed] [Google Scholar]
- 19. Neghab M, Abedini R, Soltanzadeh A, Iloon Kashkooli A, Ghayoomi SM (2012) Respiratory disorders associated with heavy inhalation exposure to dolomite dust. Iran Red Crescent Med J 14: 549–557. [PMC free article] [PubMed] [Google Scholar]
- 20. Meo SA, Al-Drees AM, Al Masri AA, Al Rouq F, Azeem MA (2013) Effect of duration of exposure to cement dust on respiratory function of non-smoking cement mill workers. Int J Environ Res Public Health 10: 390–398. 10.3390/ijerph10010390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou Y, Qing L, Zeng X, Shen Y, Zhong Y, et al. (2014) Cognitive function and plasma BDNF levels among manganese-exposed smelters. Occup Environ Med 71: 189–194. 10.1136/oemed-2013-101896 [DOI] [PubMed] [Google Scholar]
- 22. Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, et al. (2007) Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med 64: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soyseth V, Johnsen HL, Bugge MD, Hetland SM, Kongerud J (2011) Incidence of airflow limitation among employees in Norwegian smelters. Am J Ind Med 54: 707–713. 10.1002/ajim.20946 [DOI] [PubMed] [Google Scholar]
- 24. Antonini JM (2003) Health effects of welding. Crit Rev Toxicol 33: 61–103. [DOI] [PubMed] [Google Scholar]
- 25. Etemadinejad S, Mohammadian M, Alizadeh-Larimi A, Mohammadpour RA (2009) Pulmonary function in workers exposed to tobacco dust. Indian J Med Sci 63: 543–548. 10.4103/0019-5359.59987 [DOI] [PubMed] [Google Scholar]
- 26. Loukzadeh Z, Sharifian SA, Aminian O, Shojaoddiny-Ardekani A (2009) Pulmonary effects of spot welding in automobile assembly. Occup Med (Lond) 59: 267–269. 10.1093/occmed/kqp033 [DOI] [PubMed] [Google Scholar]
- 27. Johnsen HL, Hetland SM, Benth JS, Kongerud J, Soyseth V (2010) Dust exposure assessed by a job exposure matrix is associated with increased annual decline in FEV1: a 5-year prospective study of employees in Norwegian smelters. Am J Respir Crit Care Med 181: 1234–1240. 10.1164/rccm.200809-1381OC [DOI] [PubMed] [Google Scholar]
- 28. Dimich-Ward H, Beking K, DyBuncio A, Chan-Yeung M, Du W, et al. (2012) Occupational exposure influences on gender differences in respiratory health. Lung 190: 147–154. 10.1007/s00408-011-9344-x [DOI] [PubMed] [Google Scholar]
- 29. Clougherty JE (2010) A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect 118: 167–176. 10.1289/ehp.0900994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beckett WS, Pace PE, Sferlazza SJ, Perlman GD, Chen AH, et al. (1996) Airway reactivity in welders: a controlled prospective cohort study. J Occup Environ Med 38: 1229–1238. [DOI] [PubMed] [Google Scholar]
- 31. Sharifian SA, Loukzadeh Z, Shojaoddiny-Ardekani A, Aminian O (2011) Pulmonary adverse effects of welding fume in automobile assembly welders. Acta Med Iran 49: 98–102. [PubMed] [Google Scholar]
- 32. Jaen A, Zock JP, Kogevinas M, Ferrer A, Marin A (2006) Occupation, smoking, and chronic obstructive respiratory disorders: a cross sectional study in an industrial area of Catalonia, Spain. Environ Health 5: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicola ML, Carvalho HB, Yoshida CT, Anjos FM, Nakao M, et al. (2014) Young "healthy" smokers have functional and inflammatory changes in the nasal and the lower airways. Chest 145: 998–1005. 10.1378/chest.13-1355 [DOI] [PubMed] [Google Scholar]
- 34. Kessel R, Redl M, Mauermayer R, Praml GJ (1989) Changes in lung function after working with the shotcrete lining method under compressed air conditions. Br J Ind Med 46: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nemoto T, Shibata Y, Osaka D, Abe S, Inoue S, et al. (2011) Impact of cigarette smoking on maximal expiratory flows in a general population: the Takahata study. Intern Med 50: 2547–2555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.