Abstract
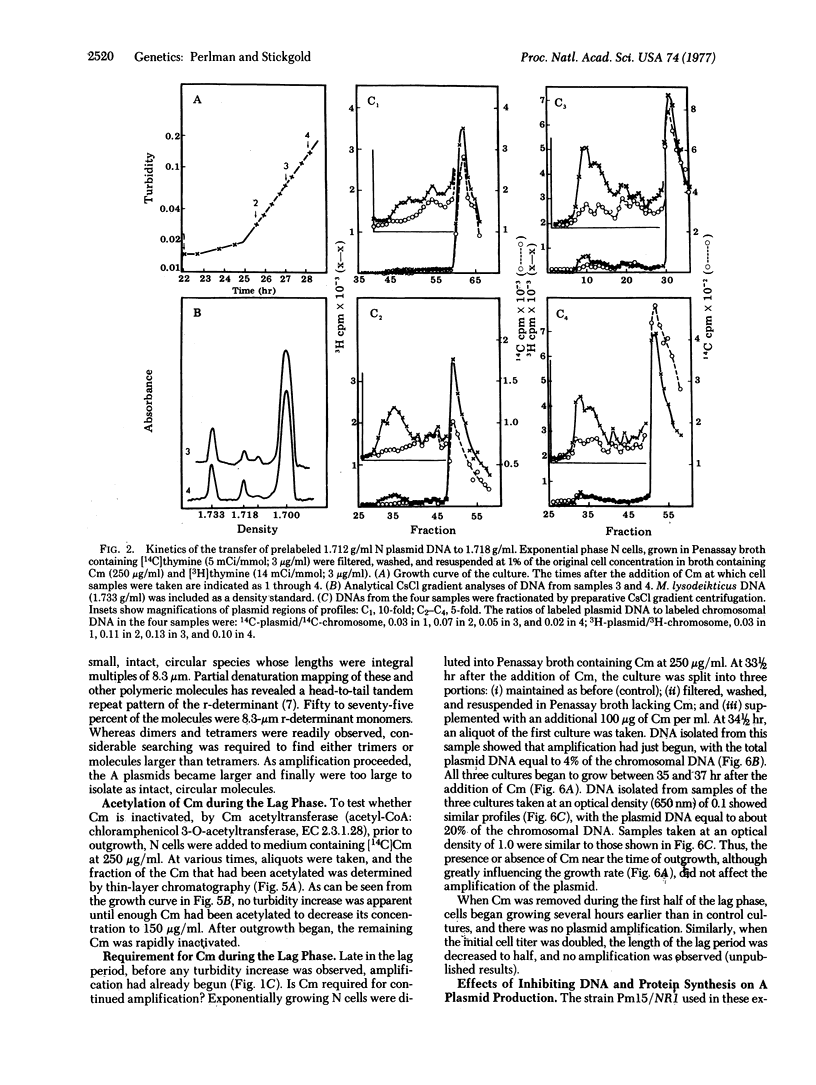
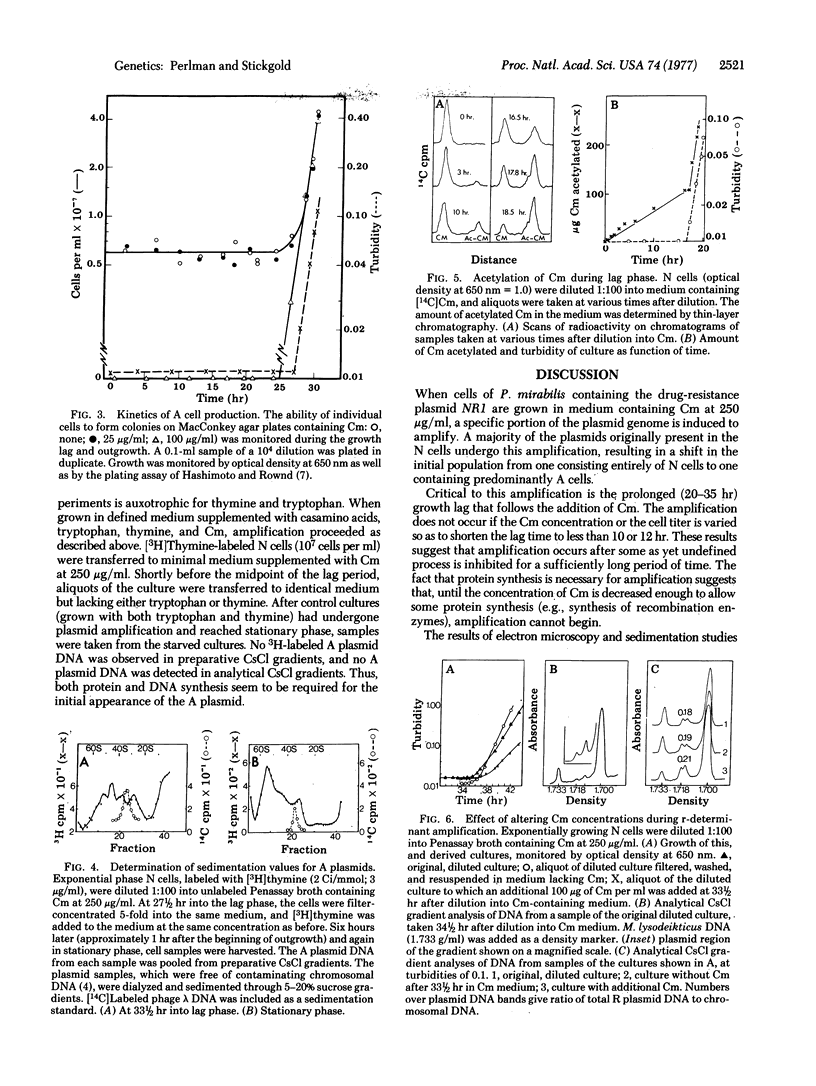
The drug-resistance plasmid, NR1, is a 37-micron circular DNA molecule that contains two components: the resistance transfer factor (29 micron) carrying the transfer genes and the genes for tetracycline resistance, and the r-determinant (8 micron) carrying the genes for resistance to several other antibiotics including chloramphenicol (Cm). In Proteus mirabilis, these two components are capable of independent replication, or they may replicate as a composite molecule. When cells of P. mirabilis containing NR1 are cultured in medium containing Cm at 250 microgram/ml a growth lag of 20-35 hr ensues. During this lag, Cm induces the selective amplification of the r-determinant, including the gene for resistance to Cm. The amplification results from the excision of the r-determinant from the R plasmid, the independent replication of the r-determinant to give polymeric as well as monomeric r-determinants, and the eventual reintegration of multiple tandem copies of the r-determinant with the resistance transfer factor to form a new R plasmid with multiple copies of the r-determinant. This mechanism represents a new level of control of gene expression in bacterial systems--namely, the induction of selective gene amplification.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N. Transposable genetic elements and plasmid evolution. Nature. 1976 Oct 28;263(5580):731–738. doi: 10.1038/263731a0. [DOI] [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Kirschner I., Goldstein R., Baran N. Physical study of prophage excision and curing of lambda prophage from lysogenic Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):703–720. doi: 10.1016/0022-2836(73)90058-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Rownd R. H. Transition of the R factor NR1 and Proteus mirabilis: level of drug resistance of nontransitioned and transitioned cells. J Bacteriol. 1975 Jul;123(1):56–68. doi: 10.1128/jb.123.1.56-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Hershberger C., Mickel S., Rownd R. Asymmetric distribution of guanine plus thymine between complementary strands of deoxyribonucleic acid of members of the Enterobacteriaceae. J Bacteriol. 1971 Apr;106(1):238–242. doi: 10.1128/jb.106.1.238-242.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Accumulation of replicating bacterial plasmid DNA during thymine limitation or hydroxyurea treatment. Mol Gen Genet. 1975 Jul 10;138(4):281–291. doi: 10.1007/BF00264797. [DOI] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Transition of R factor NR1 in Proteus mirabilis: molecular structure and replication of NR1 deoxyribonucleic acid. J Bacteriol. 1975 Sep;123(3):1013–1034. doi: 10.1128/jb.123.3.1013-1034.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Two origins of replication in composite R plasmid DNA. Nature. 1976 Jan 29;259(5541):281–284. doi: 10.1038/259281a0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Twose T. M., Holland M. J., Rownd R. H. Denaturation mapping of R factor deoxyribonucleic acid. J Bacteriol. 1975 Sep;123(3):1035–1042. doi: 10.1128/jb.123.3.1035-1042.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]


