Abstract
Between 20% and 25% of patients diagnosed with Alzheimer’s disease (AD) do not have amyloid burden as assessed by positron emission tomography imaging. Thus, there is a need for nonamyloid-directed therapies for AD, especially for those patients with non-amyloid AD. The family of phosphodiesterase-4 (PDE4) enzymes are underexploited therapeutic targets for central nervous system indications. While the PDE4A, B, and D subtypes are expressed in brain, the strict amino acid sequence conservation of the active site across the four subtypes of PDE4 has made it difficult to discover subtype inhibitors. The recent elucidation of the structure of the PDE4 N- and C-terminal regulatory domains now makes it possible to design subtype-selective, negative allosteric modulators (PDE4-NAMs). These act through closing the N-terminal UCR2 or C-terminal CR3 regulatory domains, and thereby inhibit the enzyme by blocking access of cyclic adenosine monophosphate (cAMP) to the active site. PDE4B-NAMs have the potential to reduce neuroinflammation by dampening microglia cytokine production triggered by brain amyloid, while PDE4D-NAMs have potent cognitive benefit by augmenting signaling through the cAMP/protein kinase A/cAMP response element-binding protein (CREB) pathway for memory consolidation. The importance of PDE4D for human cognition is underscored by the recent discovery of PDE4D mutations in acrodysostosis (ACRDY2: MIM 600129), an ultra rare disorder associated with intellectual disability. Thus, the family of PDE4 enzymes provides rich opportunities for the development of mechanistically novel drugs to treat neuroinflammation or the cognitive deficits in AD.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0309-7) contains supplementary material, which is available to authorized users.
Key Words: Phosphodiesterase-4, PDE4, Alzheimer’s disease, Acrodysostosis, ACRDY2, neuroinflammation
Introduction
With the aging of the world’s population, healthcare systems face an increasing burden of caring for patients with Alzheimer’s disease (AD) and other forms of dementia [1]. The economic cost of the disease is large. Typically, 2 caregivers are needed by each patient, one of whom tends to be a spouse or other family member. In the USA today, nearly 15% of Americans aged 71 years or older have dementia. This is about 3.8 million people. By 2040, that number is estimated to increase to 9.1 million [1]. The coming surge in the cost of care and number of patients affected by dementia is staggering.
Amyloid-based Therapies for AD
The discovery of disease-modifying therapies for AD currently focuses on the amyloid pathway. Deposition of amyloid plaque is a hallmark of AD pathology [2]. Genetic studies focused on Mendelian inheritance of risk for early-onset AD succeeded in identifying key elements of the amyloid pathway [3–8]. Amyloid plaque contains fibrillar aggregates of a 40- or 42-amino acid-long amyloid-β peptide known as Aβ40 or Aβ42 [2]. The Aβ peptides spontaneously form fibrillar aggregates in vitro, and appear to seed Aβ peptide aggregation when injected into the brain parenchyma [9]. Aβ42 is the more amyloidogenic of the 2 peptides. Aβ40 and Aβ42 are cleaved from a larger protein precursor known as the amyloid protein precursor (APP) [10]. APP is a Type 1 transmembrane protein. It can be processed at 1 of 3 proteolytic cleavage sites. Processing proximal to the membrane at the α-site releases the APP extracellular domain and prevents Aβ peptide formation. Processing at the β- and ϒ-sites releases the Aβ peptides. With the discovery and cloning of the APP gene, it was quickly realized that human APP mutations that increase Aβ peptide production are associated with a genetic risk of early-onset AD [3, 5, 11]. APP mutations conferring genetic risk either increase total Aβ peptide production by increasing processing at the β-site or shift processing at the ϒ-site to the more amyloidogenic Aβ42 peptide. Further genetic studies identified mutations in 2 additional genes, presenilin-1 (PS1) and presenilin-2 (PS2) as also conferring risk for early-onset AD [7]. The PS1 and PS2 mutations shift the ratio of Aβ40 to Aβ42 towards the more amyloidogenic Aβ42 peptide. Soon afterwards, a novel aspartyl protease with a signature transmembrane anchor was shown to process APP at the β-site [12–14]. The initial β-site mutations in APP increased risk for early-onset AD by increasing Aβ production [5]. Later, β-site APP mutations that decreased genetic risk for AD were found to decrease Aβ production [8]. This completed the identification of the 3 therapeutic classes for intervention in the amyloid pathway: monoclonal antibodies which—when administered systemically—deplete Aβ peptide from brain (bapineuzumab and solanuzemab), small molecule ϒ-secretase inhibitors, and small molecule β-secretase inhibitors (Fig. 1). In concert, there has been co-development of positron emission tomography (PET) imaging agents for assessing amyloid plaque burden in the brain [15]. Unfortunately, none of these therapeutics have yet shown disease modification in large, well- controlled human clinical trials. However, much has been learned.
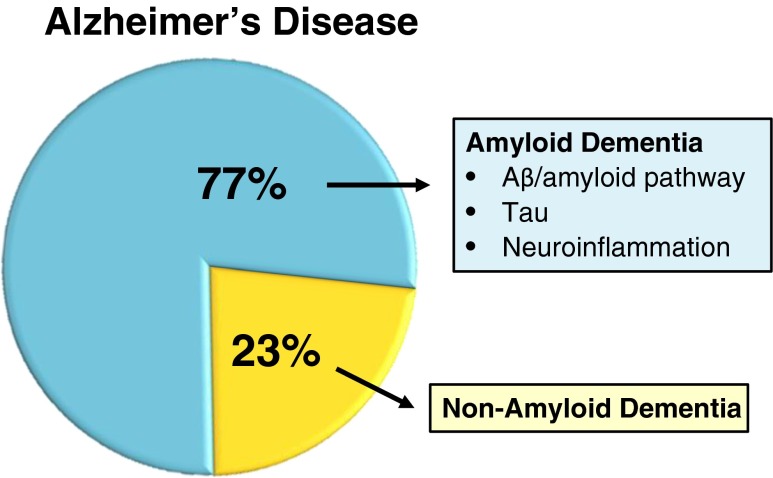
Fig. 1.
Burden of amyloid and nonamyloid dementia in patients diagnosed with Alzheimer’s disease (AD). Large human clinical trials incorporating positron emission tomography imaging of amyloid plaque reveal substantial clinical heterogeneity in amyloid burden despite a common diagnosis of AD. Development of disease-modifying therapies focuses on amyloid-β (Aβ) peptide formation and accumulation, tau neuropathy, and prevention of neuroinflammation secondary to formation of amyloid plaque. Patients with nonamyloid dementia show slow progression of cognitive impairment suggesting that cognition enhancers may have sustained benefit in that clinical subgroup
Bapineuzumab and solanuzumab were explored in multiple human phase III clinical trials in which patients were recruited on the basis of a diagnosis of AD with cognitive impairment as assessed by ≥1 clinical tests (e.g., Alzheimer’s Disease Assessment Scale-Cognitive) [16, 17]. As amyloid imaging agents became available and were used for postenrollment patient stratification, it was learned that 23 % of the patients enrolled in the bapineuzumab clinical trial and up to 25 % of the patients enrolled in the solenzanmab trial did not have amyloid plaque burden, despite the diagnosis of AD. As not all patients enrolled in the trials were studied by PET imaging, post hoc stratification by amyloid plaque burden was not possible. Neither bapineuzumab nor solenzanmab met the primary endpoint of the clinical trials—slowing disease progression. However, post hoc analysis of the bapineuzumab data suggested a positive benefit in patients treated early after diagnosis of AD [16]. Interestingly, the 23–25 % of patients with a diagnosis of AD in the absence of amyloid plaque did not show progressive deepening of cognitive impairment over the course of the 18-month study. Thus, the large bapineuzumab and solanuzumab clinical trials demonstrated that AD is a heterogeneous diagnosis and a substantial fraction of patients have nonamyloid AD with chronic but slowly progressing cognitive impairment. The two trials also showed the importance of using PET-confirmed amyloid plaque burden as a basis for enrollment of patients into clinical trials of anti-amyloid therapeutics.
Subtype-Selective PDE4 Inhibitors Provide Opportunities for Nonamyloid Therapies for AD
There is a need for the discovery of nonamyloid based therapeutics for AD. The two classes of approved drugs—acetylcholine esterase inhibitors, which address the loss of acetylcholine due to cholinergic neuron death, and memantine, a low-affinity, voltage-dependent inhibitor of the glutamatergic, N-methyl D-aspartate receptor—provide modest cognitive benefit. Given that up to 20 % of patients diagnosed with AD have nonamyloid, slowly progressing cognitive impairment, there is a need for additional drugs that address memory loss, especially in this patient subgroup (Fig. 1).
The family of phosphodiesterase-4 (PDE4) enzymes that regulate cyclic adenosine monophosphate (cAMP) signaling in neurons and glial cells represent underexploited therapeutic targets owing to the difficulty of designing compounds that are selective for one PDE4 subtype. PDE4 has 4 subtypes: PDE4A, B, C, and D. PDE4D is implicated in modulating the cAMP–protein kinase A (PKA)–cAMP response element-binding protein (CREB) pathway for memory consolidation [18, 19], while PDE4B modulates the response of monocytic cells to inflammatory stimuli [20]. Recent progress in understanding the structural basis of PDE4 regulation has made it possible to design selective PDE4D and PDE4B negative allosteric modulators (NAMs) [21, 22]. These act through modulating the closure of N- and C-terminal PDE4 regulatory domains across the active site, thereby inhibiting the enzyme (Figs 2 and 3).
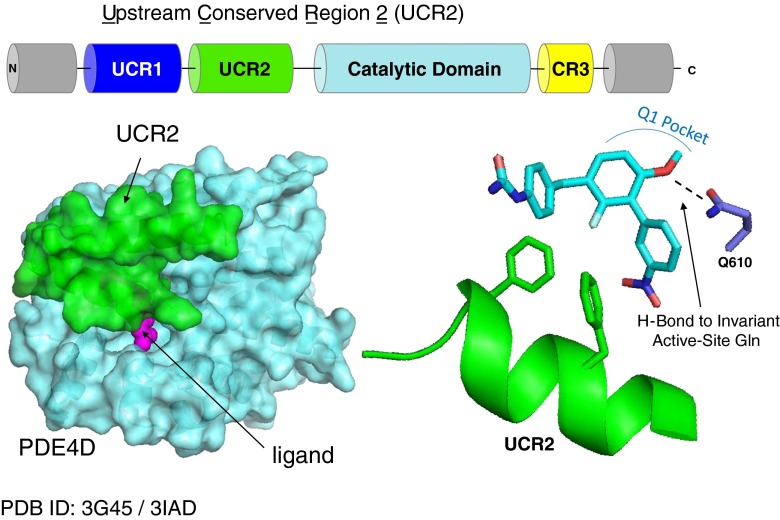
Fig. 2.
Binding mode of phosphodiesterase-4 (PDE4)D negative allosteric modulators (NAMs) directed against upstream conserved region (UCR)2. PDE4D NAMs close the UCR2 regulatory domain (green) in trans over the active site of the opposite catalytic domain (cyan) in the PDE4 dimer (only one monomer is shown), thereby preventing access by cyclic adenosine monophosphate and inhibiting the enzyme. A surface view with UCR2 closed over the active site is shown on the left with an inhibitor (purple) bound in the active site. On the right, the bound inhibitor forms a hydrogen bond to an invariant, active-site glutamine (Q610 in PDE4D7), while two arms project outwards towards UCR2. Binding of the inhibitor completes a hydrophobic interaction surface for the closure of UCR2 by displacing water from the active site. The UCR2 shown on the left is taken from a PDE4B structure (PDB ID: 3G45) as more of the regulatory domain is visible in the crystal [21]. The catalytic domain and UCR2 on the right are taken from a PDE4D structure (PDB ID: 3IAD)
Fig. 3.
Binding mode of phosphodiesterase-4 (PDE4)B negative allosteric modulators (NAMs) directed against CR3. The CR3 regulatory domain (yellow) is linked to the catalytic domain (cyan) through a short linker (gray), which allows PDE4B allosteric inhibitors to close CR3 in cis across the active site of the same monomer. PDE4B NAMs form a hydrogen bond to the invariant active site glutamine (Q615 in PDE4B3). The inhibitor captures CR3 by hydrogen bonding through a water network to lysine672. Capture of CR3 allows the amine of lysine677 (K677) to hydrogen bond to proline602 (P602) of the catalytic domain. CR3 has flexibility when closing across the PDE4B active site such that the compound selects the registration of the CR3 helix [22]
Four genes encode subtypes of mammalian PDE4, all of which contain multiple upstream exons that undergo complex splicing to generate multiple PDE4 isoforms that differ in N-terminal amino acid sequence [23–26]. The complex pattern of splice isoforms allows subtypes of PDE4 to be targeted to different cellular microdomains and for isoform- and subtype-selective regulation of PDE4 activity by accessory proteins [27]. PDE4A, B, and D are expressed in brain [28–32].
Rolipram is a widely used PDE4 inhibitor that has been evaluated in multiple models of learning and memory [33]. However, the compound has <10-fold selectivity for different PDE4 isoforms [21]. Rolipram reverses memory deficits in mouse models of AD and provides cognitive benefit in rodent models of stroke [34], traumatic brain injury [35], and Huntington’s disease [36–40]. Gong et al. have shown in 3-month old APP/PS1 transgenic mice that rolipram reverses deficits in hippocampal long-term potentiation, contextual fear conditioning, and the Morris water maze [37]. In the same study, treatment with rolipram for 3 weeks showed persistent benefit 3 months later in 6-month-old APP/PS1 transgenic mice [41]. Thus, rolipram appeared to delay or reverse disease progression in the APP/PS1 transgenic model, while no effect was found on the Aβ load. In a subsequent study, Smith et al. showed that there is loss of dendritic spines and dystrophic neurites in APP/PS1 transgenic mice and in human AD [42]. In the model, rolipram reverses acute alterations in dendritic spines caused by Aβ peptide, as well as the chronic loss of spines in APP/PS1 transgenic mice. Thus, treatment with a PDE4 inhibitor may work independently of therapies aimed at lowering Aβ peptide levels in brain. It has also been shown that rolipram reverses memory deficits induced by injection of Aβ 1-40 or 25–35 into rat hippocampus CAI [39, 40].
PDE4B in Neuroinflammation Secondary to Amyloid Plaque Formation
The benefit of rolipram in AD models may be owing, in part, to inhibition of PDE4B with consequent reduction of neuroinflammation. Recent studies in patients with AD have used PET imaging with 11C-PBR28 to measure neuroinflammation [43]. An increase in 11C-PBR28 binding correlates with amyloid burden and with the degree of cognitive impairment. PDE4B is induced in monocytes, macrophages and microglial cells by inflammatory stimuli including Aβ-peptides [39, 44–47]. Mice deficient in PDE4B display reduced tumor necrosis factor-α production in response to an inflammatory stimulus, while PDE4B NAMs similarly blunt monocytic cell production of tumor necrosis factor-α in vitro and in vivo [48, 49]. Thus, PDE4B represents a novel therapeutic target that may reduce neuroinflammation in AD.
Exploitation of PDE4B as a target has been difficult as there are no amino acid sequence differences in the active site that distinguish PDE4B from the other PDE4 subtypes [50]. More recently, highly selective PDE4B inhibitors have been reported [48, 49]. These act through engaging the C-terminal CR3 regulatory domain through a water network and thereby closing the active site to access by cAMP [22] (Fig. 3).
Genetic Validation of the Role of PDE4D in Human Cognition
The first learning mutation described in fruit flies, dunce, is a deletion of the single Drosophila PDE4 gene [51–53]. Studies of mice deficient in PDE4D generally indicate that the enzyme plays a role in modulating memory formation [54]. Li et al. found that mice deficient in PDE4D display memory enhancement in the radial arm maze, water maze, and object recognition tests [55]. These effects are mimicked in wild-type mice by treatment with rolipram or PDE4D inhibitors [21, 56].
Furthermore, bilateral microinfusion into the hippocampal dentate gyrus of lentiviral vectors that produce silencing RNAs targeting long PDE4D isoforms, downregulates PDE4D4 and PDE4D5, enhances memory, and increases hippocampal neurogenesis and CREB phosphorylation [55]. Thus, PDE4D regulates the PKA/CREB pathway in hippocampal neurons, which is important for memory formation. This is a key finding that supports the development of PDE4D-NAMs for improving cognition.
Sequence comparison of the 4 PDE4 genes revealed that the PDE4 subfamily differs from other phosphodiesterases owing to the presence of 2 upstream conserved regions (UCR1 and UCR2) [23, 57]. These were shown by the Conti and Houslay laboratories to be key regulatory domains that control the activation of the enzyme by PKA [58, 59]. Long PDE4 isoforms contain both UCR1 and UCR2, while short and super short isoforms contain only UCR2 (Fig. 2). UCR1 is the target of PKA phosphorylation, which regulates whether the enzyme is in a fully activated form or a partially inhibited basal state. The short and super-short isoforms are fully active owing to the absence of UCR1, and instead their activity level in the cell is regulated transcriptionally [31]. UCR1 and UCR2 form an oligomerization domain needed for dimerization and regulate the opening and closing of UCR2 across the active site [60, 61]. UCR2 closes in trans- across the active site, such that one active site is fully closed and the other is partially active [21].
The genetic relevance of the UCR1/UCR2 regulatory mechanism is supported by the recent discovery of human PDE4D mutations in acrodysostosis (ACRDY2; MIM 600129) [62–65]. ACRDY2 is a developmental disorder that causes intellectual disability, short stature, facial dysmorphia, and brachydactyly. Most ACRDY2 mutations are spontaneous, but one instance of inheritance from a parent to 2 offspring has been reported [64]. Intelligence is impaired in 90 % of affected children with intelligence quotients ranging from 50 to 80 [66]. Without exception, the ACRDY2 mutations are missense mutations. Amino acid substitutions avoid the active site, and instead cluster in UCR1 or affect contact residues between UCR1 and UCR2, or UCR2 and the PDE4D catalytic domain [64] (Fig. 4). That UCR1 is one of the hotspots of mutation in ACRDY2 indicates the biological importance of the long forms of PDE4D over the short and super-short forms that lack UCR1.
Fig. 4.
Proposed phosphodiesterase-4 (PDE4)D dimer with the location of acrodyostosis (ACRDY2) mutations. Richter and Conti previously showed that a C-terminal helix of upstream conserved region (UCR)1 and an N-terminal helix of UCR2 (neither of which are visible in our co-crystal structures) are needed for dimerization of PDE4D (cartoon, top) [60, 61]. Three views of the proposed PDE4D dimer are shown. The “TOP” and “FACE” views show how UCR2 closes in trans, where the cyan protein’s UCR2 closes over the green protein’s catalytic domain and vice versa. The two active sites face outwards in opposite directions from the dimer. The dimer interface occurs across the crystallographic unit cell, and is supported by the finding of Lee et al. that mutation of aspartate466 to arginine (D466R) and arginine502 to aspartic acid (R502D) disrupt dimerization of the PDE4D catalytic domain (purple) [67]. The ACRDY2 mutations are shown in red. These occur primarily on contact surfaces between UCR2 and the catalytic domain (V268A and I272V on UCR2, which are hidden in the surface view, and G612D, Y616H and I617T on the opposite catalytic domain surface). In addition, there are 4 “hot spots” for ACRDY2 mutations on the top of the PDE4D dimer (red circles, “TOP VIEW”). These occur on a surface parch of the catalytic domain (T526P, M527V/I, and E529A) and at the N-terminus of the second UCR2 helix (M242V and A243V). No ACRDY2 mutations have been discovered in the active site, on the dimerization surface between the catalytic domains (purple), or on the underside of the PDE4D dimer (“BOTTOM VIEW”). The Richter and Conti [60], and Lee et al. [67] dimer proposals are consistent with a crystal structure of the PDE4B dimer that has been obtained by Cedervall and Pandit (personal communication)
PDE4 plays a critical role in the spatial and temporal patterning of signaling within cAMP microdomains [68–71]. The Drosophila dunce mutation, a PDE4-null mutation, disrupts learning and memory by causing dysregulation of spatially restricted cAMP signaling in mushroom body neurons [72]. In acrodysostosis, ACRDY2 mutations are thought to cause activation of the enzyme [64], thereby reducing cAMP signaling in pathways important for memory formation. The importance of PDE4 for regulating spatial and temporal patterns of cAMP signaling in neurons has been shown in mouse cortical neurons by using cAMP biosensors to image cAMP flux in living cells [73].
Molecular Pharmacology of PDE4D NAMs in Models of Learning and Memory
It is possible to design small molecule negative allosteric modulators of PDE4D that act through closing UCR2 over the active site [21]. Indeed, rolipram inhibits PDE4 through that mechanism. What has been known as the high-affinity rolipram binding site is actually the PDE4 conformation with UCR2 closed [21]. The low-affinity rolipram binding site measures rolipram binding in the open active site without the closure of UCR2. Rolipram has little selectivity for different PDE4 subtypes. However, a key amino acid polymorphism in primates allows the design of PDE4D subtype-selective NAMs. PDE4D in primates is distinguished from PDE4A, B, and C by a phenylalanine in UCR2 which orients towards the cAMP pyrimidine when UCR2 is closed, while PDE4A, B, and C contain a tyrosine. Rolipram binding accommodates closure of UCR2 containing either the phenylalanine or tyrosine, while PDE4D subtype inhibitors achieve selectivity through steric clash with UCR2 containing a tyrosine that projects a hydroxyl group deeper into the active site. The tyrosine/phenylalanine difference in UCR2 accounts for the difference in affinity towards substrate for PDE4D compared with PDE4B [21]. Subtype selectivity is not achievable with PDE4 inhibitors that bind in the active site competitively with cAMP independently of UCR2 as there are no amino acid sequence differences in the active site among the PDE4 subtypes [74].
PDE4D NAMs have been shown to have cognitive benefit in models of cholinergic deficit (scopolamine-impaired Y-maze) and in normal rats and mice (novel object recognition) [21]. More recently, PDE4D NAMs have been shown to improve cognitive performance in healthy adult monkeys (object reaching task) [75]. A known issue with rolipram is the lack of tolerability in multiple species due to nausea and vomiting, a common problem with all classes of PDE4 inhibitors. PDE4D NAMs, in contrast, show improved tolerability in multiple species as they only partially inhibit the enzyme [21, 75].
Conclusions
There is a need for the development of nonamyloid based therapies for AD. The availability of PET agents for imaging amyloid plaque has revealed that 20–25 % of patients diagnosed with AD do not have amyloid plaque and that in these patients there is slow progression of cognitive impairment. PDE4B NAMs have the potential to address neuroinflammation secondary to the formation of amyloid plaque in AD, while PDE4D NAMs may improve cognitive impairment. Molecular genetic studies of learning and memory in model organisms have shown the importance of the cAMP/PKA/CREB pathway for long-term memory. The importance of this pathway in humans receives genetic validation through the identification of PDE4D mutations in acrodysostosis (ACRDY2), an ultra rare genetic disorder that causes intellectual disability. PDE4D NAMs that modulate enzyme activity by closing the UCR2 regulatory domain over the active site, thereby inhibiting the enzyme, offer a novel mechanism for addressing cognitive impairment in AD and other neurologic and psychiatric conditions.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgments
This study was funded by the National Institute of Neurological Disorders and Stroke award U01NS078034 and by the National Institute of Mental Health award 5R43MH091791 to M.E.G.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 3.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 4.Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 5.Mullan M, Crawford F, Axelman K, et al. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 6.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 7.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 9.Pangalos MN, Jacobsen SJ, Reinhart PH. Disease modifying strategies for the treatment of Alzheimer's disease targeted at modulating levels of the beta-amyloid peptide. Biochem Soc Trans. 2005;33:553–558. doi: 10.1042/BST0330553. [DOI] [PubMed] [Google Scholar]
- 10.Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 12.Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 14.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 15.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 16.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 18.Li YF, Cheng YF, Huang Y, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 20.Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- 21.Burgin AB, Magnusson OT, Singh J, et al. Design of phosphodiesterase type 4D (PDE4D) allosteric modulators for cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- 22.Fox D, 3rd, Burgin AB, Gurney ME. Structural basis for the design of selective phosphodiesterase 4B inhibitors. Cell Signal. 2014;26:657–663. doi: 10.1016/j.cellsig.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolger G, Michaeli T, Martins T, et al. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swinnen JV, Joseph DR, Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989;86:5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monaco L, Vicini E, Conti M. Structure of two rat genes coding for closely related rolipramsensitive cAMP phosphodiesterases. Multiple mRNA variants originate from alternative splicing and multiple start sites. J Biol Chem. 1994;269:347–357. [PubMed] [Google Scholar]
- 27.Houslay MD, Baillie GS, Maurice DH. cAMP-specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 28.Mori F, Perez-Torres S, De Caro R, et al. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat. 2010;40:36–42. doi: 10.1016/j.jchemneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and [3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/S0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 30.Miro X, Perez-Torres S, Puigdomenech P, Palacios JM, Mengod G. Differential distribution of PDE4D splice variant mRNAs in rat brain suggests association with specific pathways and presynaptical localization. Synapse. 2002;45:259–269. doi: 10.1002/syn.10100. [DOI] [PubMed] [Google Scholar]
- 31.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–811. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. doi: 10.1002/(SICI)1096-9861(19990503)407:2<287::AID-CNE9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imanishi T, Sawa A, Ichimaru Y, et al. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321:273–278. doi: 10.1016/S0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- 35.Titus DJ, Sakurai A, Kang Y, et al. Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury. J Neurosci. 2013;33:5216–5226. doi: 10.1523/JNEUROSCI.5133-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeMarch Z, Giampa C, Patassini S, Bernardi G, Fusco FR. Beneficial effects of rolipram in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis. 2008;30:375–387. doi: 10.1016/j.nbd.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Yang XM, Zhuo YY, et al. The phosphodiesterase-4 inhibitor rolipram reverses Abeta-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol. 2012;15:749–766. doi: 10.1017/S1461145711000836. [DOI] [PubMed] [Google Scholar]
- 40.Cheng YF, Wang C, Lin HB, et al. Inhibition of phosphodiesterase-4 reverses memory deficits produced by Abeta25-35 or Abeta1-40 peptide in rats. Psychopharmacology. 2010;212:181–191. doi: 10.1007/s00213-010-1943-3. [DOI] [PubMed] [Google Scholar]
- 41.Gong B. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliva AA, Jr, Kang Y, Furones C, et al. Phosphodiesterase isoform-specific expression induced by traumatic brain injury. J Neurochem. 2012;123:1019–1029. doi: 10.1111/jnc.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh M, Garcia-Castillo D, Aguirre V, et al. Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia. 2012;60:1839–1859. doi: 10.1002/glia.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebastiani G, Morissette C, Lagace C, et al. The cAMPspecific phosphodiesterase 4B mediates Abeta-induced microglial activation. Neurobiol Aging. 2006;27:691–701. doi: 10.1016/j.neurobiolaging.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Naganuma K, Omura A, Maekawara N, et al. Discovery of selective PDE4B inhibitors. Bioorg Med Chem Lett. 2009;19:3174–3176. doi: 10.1016/j.bmcl.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 49.Goto T, Shiina A, Yoshino T, et al. Identification of the fused bicyclic 4-amino-2-phenylpyrimidine derivatives as novel and potent PDE4 inhibitors. Bioorg Med Chem Lett. 2013;23:3325–3328. doi: 10.1016/j.bmcl.2013.03.104. [DOI] [PubMed] [Google Scholar]
- 50.Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233–2247. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 53.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce + gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986;83:9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YF, Cheng YF, Huang Y, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruno O, Fedele E, Prickaerts J, et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–2063. doi: 10.1111/j.1476-5381.2011.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis RL, Takayasu H, Eberwine M, Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce + gene. Proc Natl Acad Sci U S A. 1989;86:3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann R, Wilkinson IR, McCallum JF, Engels P, Houslay MD. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: generation of a molecular model. Biochem J. 1998;333(Pt 1):139–149. doi: 10.1042/bj3330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter W, Conti M. Dimerization of the type 4 cAMP-specific phosphodiesterases is mediated by the upstream conserved regions (UCRs) J Biol Chem. 2002;277:40212–40221. doi: 10.1074/jbc.M203585200. [DOI] [PubMed] [Google Scholar]
- 61.Richter W, Conti M. The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterases. J Biol Chem. 2004;279:30338–30348. doi: 10.1074/jbc.M312687200. [DOI] [PubMed] [Google Scholar]
- 62.Lee H, Graham JM, Jr, Rimoin DL, et al. Exome sequencing identifies PDE4D mutations in acrodysostosis. Am J Hum Genet. 2012;90:746–751. doi: 10.1016/j.ajhg.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linglart A, Fryssira H, Hiort O, et al. PRKAR1A and PDE4D mutations cause acrodysostosis but two distinct syndromes with or without GPCR-signaling hormone resistance. J Clin Endocrinol Metab. 2012;97:E2328–E2338. doi: 10.1210/jc.2012-2326. [DOI] [PubMed] [Google Scholar]
- 64.Lynch DC, Dyment DA, Huang L, et al. Identification of novel mutations confirms Pde4d as a major gene causing acrodysostosis. Hum Mutat. 2013;34:97–102. doi: 10.1002/humu.22222. [DOI] [PubMed] [Google Scholar]
- 65.Michot C, Le Goff C, Goldenberg A, et al. Exome sequencing identifies PDE4D mutations as another cause of acrodysostosis. Am J Hum Genet. 2012;90:740–745. doi: 10.1016/j.ajhg.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler MG, Rames LJ, Wadlington WB. Acrodysostosis: report of a 13-year-old boy with review of literature and metacarpophalangeal pattern profile analysis. Am J Med Genet. 1988;30:971–980. doi: 10.1002/ajmg.1320300416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee ME, Markowitz J, Lee JO, Lee H. Crystal structure of phosphodiesterase 4D and inhibitor complex(1) FEBS Lett. 2002;530:53–58. doi: 10.1016/S0014-5793(02)03396-3. [DOI] [PubMed] [Google Scholar]
- 68.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol. 2007;580:765–776. doi: 10.1113/jphysiol.2006.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terrin A, Di Benedetto G, Pertegato V, et al. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gervasi N, Tchenio P, Preat T. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron. 2010;65:516–529. doi: 10.1016/j.neuron.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Castro LR, Gervasi N, Guiot E, et al. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J Neurosci. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Peng MS, Chen Y, et al. Structures of the four subfamilies of phosphodiesterase-4 provide insight into the selectivity of their inhibitors. Biochem J. 2007;408:193–201. doi: 10.1042/BJ20070970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutcliffe JS, Beaumont V, Watson JM, et al. Efficacy of selective PDE4D negative allosteric modulators in the object retrieval task in female cynomolgus monkeys (Macaca fascicularis) PLoS One. 2014;9:e102449. doi: 10.1371/journal.pone.0102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)