Abstract
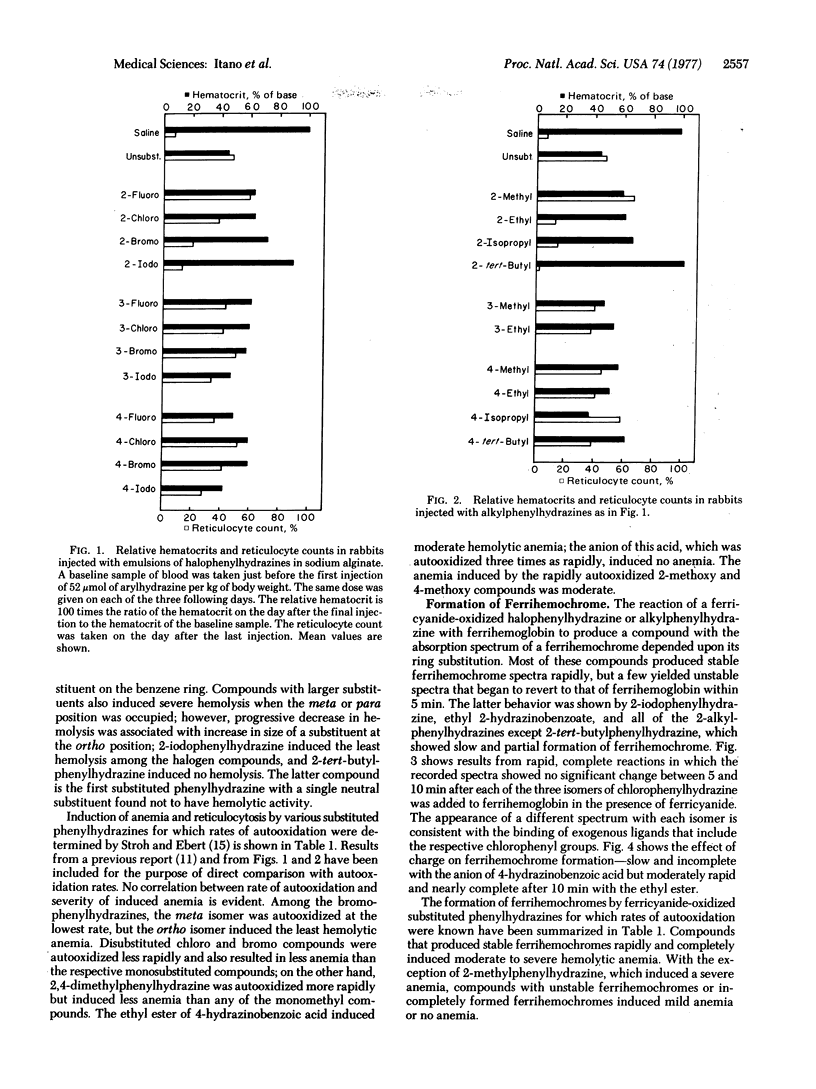
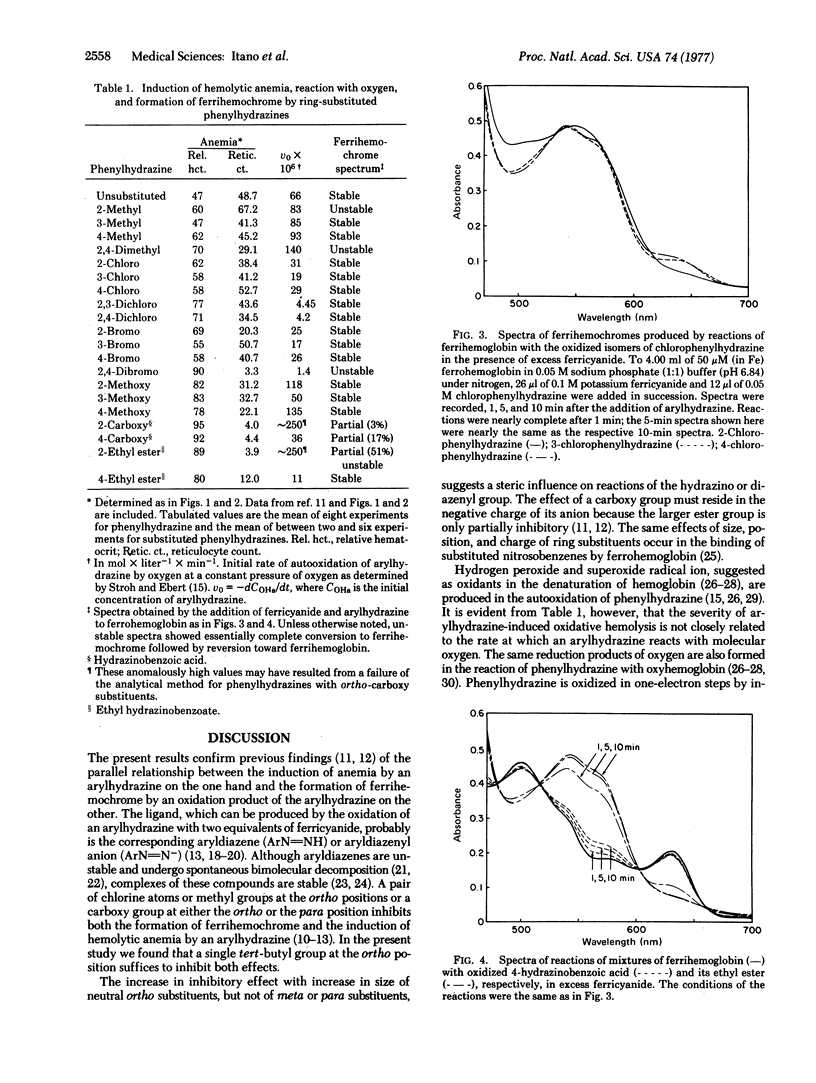
We have investigated the effect of size of a single neutral ring substituent on the induction of hemolytic anemia and the formation of a ferrihemochrome by substituted phenylhydrazines. The severity of induced anemia decreased with increase in size of a halogen atom or an alkyl group ortho to the hydrazino group, little anemia resulting from 2-iodophenylhydrazine and no anemia from 2-tert-butylphenylhydrazine. The size of a halogen atom or an alkyl group at the meta or para position had relatively little effect on the severity of induced anemia. The ability of an arylhydrazine to induce hemolytic anemia paralleled its ability to produce a ferrihemochrome with an exogenous ligand, probably the corresponding aryldiazene. In general, rapid and complete formation of ferrihemochrome occurred with arylhydrazines that induced severe anemia. The degree of hemolysis induced by an arylhydrazine was not related to its rate of autooxidation, i.e., the rate at which oxidants are formed by the reduction of oxygen. We propose a mechanism of arylhydrazine-induced oxidative denaturation based on the simultaneous formation of hydroxyl radical and aryldiazene ferrihemochrome in a reaction of oxyhemoglobin with arylhydrazine. We suggest that after oxidation of the porphyrin ring is initiated by a hydroxyl radical, oxidative cleavage of the ring is facilitated by the presence of a large ligand in the heme crevice. Thus, aryldiazene ferrihemochrome may contribute to instability in a hemoglobin molecule, whereas globin ferrihemochrome results from instability.
Keywords: arylhydrazine, aryldiazene, hemoglobin, oxidative denaturation, Heinz body anemia
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- BEAVEN G. H., WHITE J. C. Oxidation of phenylhydrazines in the presence of oxyhaemoglobin and the origin of Heinz bodies in erythrocytes. Nature. 1954 Feb 27;173(4400):389–391. doi: 10.1038/173389b0. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., BALUDA M. C. The role of methemoglobin in oxidative degradation of hemoglobin. Acta Haematol. 1962;27:321–333. doi: 10.1159/000206813. [DOI] [PubMed] [Google Scholar]
- Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969 Mar;21(1):73–103. [PubMed] [Google Scholar]
- Bonnett R., Dimsdale M. J. The meso-reactivity of porphyrins and related compounds. V. The meso-oxidation of metalloporphyrins. J Chem Soc Perkin 1. 1972;20:2540–2548. doi: 10.1039/p19720002540. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H. The unstable haemoglobin haemolytic anaemias. Semin Hematol. 1969 Apr;6(2):116–132. [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Loe R. S., Anderson C. M., Moffat K. Structure of cyanide methemoglobin. J Mol Biol. 1976 Jul 5;104(3):687–706. doi: 10.1016/0022-2836(76)90129-7. [DOI] [PubMed] [Google Scholar]
- FERTMAN M. H., FERTMAN M. B. Toxic anemias and Heinz bodies. Medicine (Baltimore) 1955 May;34(2):131–192. doi: 10.1097/00005792-195505000-00001. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Stern A., Peisach J. The mechanism of superoxide anion generation by the interaction of phenylhydrazine with hemoglobin. J Biol Chem. 1976 May 25;251(10):3045–3051. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The generation of O2-by the interaction of the hemolytic agent, phenylhydrazine, with human hemoglobin. J Biol Chem. 1975 Mar 25;250(6):2401–2403. [PubMed] [Google Scholar]
- Goldstein G. W., Hammaker L., Schmid R. The catabolism of Heinz bodies: an experimental model demonstrating conversion to non-bilirubin catabolites. Blood. 1968 Mar;31(3):388–395. [PubMed] [Google Scholar]
- Gordon-Smith E. C., White J. M. Oxidative haemolysis and Heinz body haemolytic anaemia. Br J Haematol. 1974 Apr;26(4):513–517. doi: 10.1111/j.1365-2141.1974.tb00494.x. [DOI] [PubMed] [Google Scholar]
- HARLEY J. D., MAUER A. M. Studies on the formation of Heinz bodies. II. The nature and significance of Heinz bodies. Blood. 1961 Apr;17:418–433. [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Hirota K., Hosokawa K. Mechanism of induction of haemolytic anaemia by phenylhydrazine. Nature. 1975 Aug 21;256(5519):665–667. doi: 10.1038/256665a0. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Hollister D. W., Fogarty W. M., Jr, Mannen S. Effect of ring substitution on the hemolytic action of arylhydrazines. Proc Soc Exp Biol Med. 1974 Dec;147(3):656–658. doi: 10.3181/00379727-147-38409. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Hosokawa K., Hirota K. Induction of haemolytic anaemia by substituted phenylhydrazines. Br J Haematol. 1976 Jan;32(1):99–104. doi: 10.1111/j.1365-2141.1976.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Itano H. A., Mannen S. Reactions of phenyldiazene and ring-substituted phenyldiazenes with ferrihemoglobin. Biochim Biophys Acta. 1976 Jan 14;421(1):87–96. doi: 10.1016/0304-4165(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Itano H. A. Phenyldiimide, hemoglobin, and Heinz bodies. Proc Natl Acad Sci U S A. 1970 Oct;67(2):485–492. doi: 10.1073/pnas.67.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. G., Ibers J. A. Crystal and molecular structure of the free base porphyrin, mesoporphyrin IX dimethyl ester. J Am Chem Soc. 1975 Sep 17;97(19):5363–5369. doi: 10.1021/ja00852a008. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry. 1976 Feb 10;15(3):681–687. doi: 10.1021/bi00648a036. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Colleran E. Methine bridge selectivity in haem-cleavage reactions: relevance to the mechanism of haem catabolism. Biochem Soc Trans. 1976;4(2):209–214. doi: 10.1042/bst0040209. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Rachmilewitz E. A. The demonstration of ferrihemochrome intermediates in heinz body formation following the reduction of oxyhemoglobin A by acetylphenylhydrazone. Biochim Biophys Acta. 1975 Jun 26;393(2):404–418. doi: 10.1016/0005-2795(75)90069-0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. The Croonian Lecture, 1968. The haemoglobin molecule. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):113–140. doi: 10.1098/rspb.1969.0043. [DOI] [PubMed] [Google Scholar]
- ROSTORFER H. H., CORMIER M. J. The formation of hydrogen peroxide in the reaction of oxyhemoglobin with methemoglobin-forming agents. Arch Biochem Biophys. 1957 Sep;71(1):235–249. doi: 10.1016/0003-9861(57)90025-5. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Blumberg W. E. Studies on the stability of oxyhemoglobin A and its constituent chains and their derivatives. J Biol Chem. 1971 May 25;246(10):3356–3366. [PubMed] [Google Scholar]
- Rentsch G. Genesis of Heinz bodies and methemoglobin formation. Biochem Pharmacol. 1968 Mar;17(3):423–427. doi: 10.1016/0006-2952(68)90252-9. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- STRYER L., KENDREW J. C., WATSON H. C. THE MODE OF ATTACHMENT OF THE AZIDE ION TO SPERM WHALE METMYOGLOBIN. J Mol Biol. 1964 Jan;8:96–104. doi: 10.1016/s0022-2836(64)80152-2. [DOI] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Slater L. M., Muir W. A., Weed R. I. Influence of splenectomy on insoluble hemoglobin inclusion bodies in beta thalassemic erythrocytes. Blood. 1968 Jun;31(6):766–777. [PubMed] [Google Scholar]
- Wallace W. J., Caughey W. S. Mechanism for the autoxidation of hemoglobin by phenols, nitrite and "oxidant" drugs. Peroxide formation by one electron donation to bound dioxygen. Biochem Biophys Res Commun. 1975 Feb 3;62(3):561–567. doi: 10.1016/0006-291x(75)90435-0. [DOI] [PubMed] [Google Scholar]
- Wallace W. J., Maxwell J. C., Caughey W. S. The mechanisms of hemoglobin autoxidation. Evidence for proton-assisted nucleophilic displacement of superoxide by anions. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1104–1110. doi: 10.1016/0006-291x(74)90810-9. [DOI] [PubMed] [Google Scholar]
- Wennberg E., Weiss L. Splenic erythroclasia: an electron microscopic study of hemoglobin H disease. Blood. 1968 Jun;31(6):778–790. [PubMed] [Google Scholar]
- White J. M., Dacie J. V. The unstable hemoglobins--molecular and clinical features. Prog Hematol. 1971;7(0):69–109. [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]


