Abstract
Introduction
Frailty is a major concern due to its costly and widespread consequences, yet evidence of effective interventions to delay or reduce frailty is lacking. Our previous study found that a multifactorial intervention was feasible and effective in reducing frailty in older people who were already frail. Identifying and treating people in the pre-frail state may be an effective means to prevent or delay frailty. This study describes a randomised controlled trial that aims to evaluate the effectiveness of a multifactorial intervention on development of frailty in older people who are pre-frail.
Methods and analysis
A single centre randomised controlled trial with concealed allocation, assessor blinding and intention-to-treat analysis. Two hundred and thirty people aged above 70 who meet the Cardiovascular Health Study frailty criteria for pre-frailty, reside in the community and are without severe cognitive impairment will be recruited. Participants will be randomised to receive a multifactorial intervention or usual care. The intervention group will receive a 12-month interdisciplinary intervention targeting identified characteristics of frailty and problems identified during geriatric assessment. Participants will be followed for a 12-month period. Primary outcome measures will be degree of frailty measured by the number of Cardiovascular Health Study frailty criteria present, and mobility measured with the Short Physical Performance Battery. Secondary outcomes will include measures of mobility, mood and use of health and community services.
Ethics and dissemination
The study was approved by the Northern Sydney Local Health District Health Research Ethics Committee (1207-213M). The findings will be disseminated through scientific and professional conferences, and in peer-reviewed journals.
Trial registration number
Australian New Zealand Clinical Trials Registry: ACTRN12613000043730.
Keywords: GERIATRIC MEDICINE, frail elderly, randomised trial
Strengths and limitations of this study.
First randomised controlled trial to evaluate the effectiveness of an intervention on the development of frailty in older people who are pre-frail.
Randomised controlled trial with blinded assessors and intention-to-treat analysis.
Generalisable to community-dwelling pre-frail older people; there is an objective measure of pre-frailty and minimal exclusion criteria. The intervention being examined is readily transferable to routine clinical practice in the aged care health service setting and the interdisciplinary approach is relevant to several professional groups in aged care.
Lack of blinding of participants and staff delivering the intervention due to the nature of the intervention.
Introduction
Intervention to prevent or delay frailty has important benefits for older people, health services and society.1 2 Frailty is a medical syndrome with numerous causes, characterised by reduced strength, endurance and physiological function, resulting in increased vulnerability to functional decline, dependence and/or death.1 Pre-frailty is an intermediate stage between non-frail and frail. Identifying and treating people in the pre-frail state may be an effective way to prevent or delay frailty.
Frailty can be defined using the Cardiovascular Health Study (CHS) frailty phenotype3 which contains five criteria (unexplained weight loss, weakness, low activity, exhaustion and slowness) that reflect underlying dysregulation in multiple physiological processes.4 People are classified as non-frail if they meet no criteria, pre-frail if they meet one or two criteria, and frail if they meet three or more criteria. The frailty phenotype is predictive of falls, disability, institutionalisation, hospitalisation and mortality; pre-frail individuals have a significantly higher risk of developing these adverse outcomes than non-frail people, and frail individuals have a still higher risk.3 Pre-frailty and frailty are common; a recent systematic review found that the prevalence of pre-frailty (as defined by the frailty phenotype) in community-dwelling people aged 65 years or older was 38–53% (mean 44.2%), and the prevalence of frailty was 4–17% (mean 9.9%).5 As the proportion of older people is rising globally, the costs associated with frailty will increase in the future. Preventing or delaying frailty has the potential to reduce the burden on individuals and society.
Research into interventions to prevent or reduce frailty is in its infancy. While studies have found that outcomes for frail older people can be improved using multifactorial interventions such as comprehensive geriatric assessment, and single interventions including exercise programmes,6 nutritional supplementation and reduction of polypharmacy,1 the effect of intervention on frailty itself is seldom examined. Our recent randomised trial evaluated the effect of a multifactorial interdisciplinary intervention on frailty as a primary outcome (measured using the frailty phenotype), and found that the intervention significantly reduced frailty in frail community-dwelling older people.7
Implementing interventions for pre-frail older people may prevent the development of frailty. Older people transition between frailty states,8 and pre-frail individuals have more than twice the risk of becoming frail compared with non-frail people.3 Transition from pre-frail to frail often ensues from an acute medical event or a psychological stress exceeding the person's capacity for recovery.9 Intervention to increase reserve capacity and reduce the impact of potential stressors may therefore reduce the risk of becoming frail. Evidence suggests that pre-frail older people may respond better to intervention than people who have already moved to a frail state,10 11 and because pre-frail people have significantly less disability than frail people3 there is potential for more intensive interventions.
Few trials have identified and targeted pre-frail participants. Previous trials have included samples that are probably pre-frail, for example people at risk of falling;12 however, studies need to have pre-frailty as an inclusion criterion for results to be generalisable to this population. Recent randomised trials10 13 14 and an observational study15 have investigated the effects of exercise in people defined as pre-frail using the frailty phenotype; exercise appears to improve function in pre-frail people; however, larger studies are needed. To the best of our knowledge, no intervention has been developed to specifically prevent the transition to frailty in pre-frail older people.
We plan to conduct the Pre-Frailty Intervention Trial (Pre-FIT), a randomised controlled trial that aims to determine whether delivering a multifactorial, interdisciplinary intervention to older people who are pre-frail prevents progression to frailty and improves mobility. We will implement a modification of the intervention previously found to reduce frailty and improve mobility in frail older people16 to determine whether pre-frail participants receive similar benefits with respect to frailty levels and mobility. To the best of our knowledge, this will be the first study to examine the effects of an intervention specifically targeting the degree of frailty among older people who are pre-frail. The primary research question is: Does the multifactorial interdisciplinary intervention prevent the progression to frailty (assessed with a frailty phenotype score) and improve mobility among pre-frail older people, when compared with usual care?
Methods and design
Design
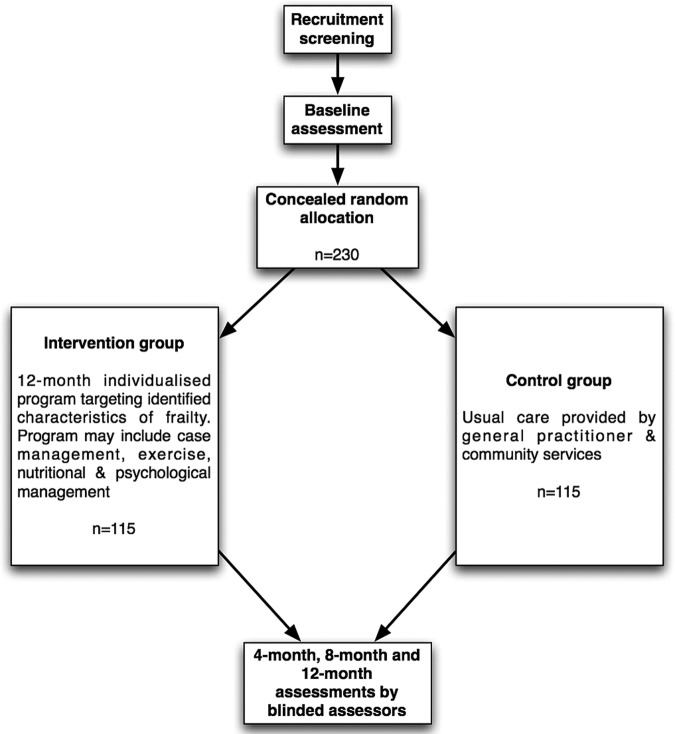
A randomised controlled trial will be conducted among 230 participants who are pre-frail. Figure 1 gives an overview of the study design. All participants will give written informed consent prior to randomisation (see online supplementary appendix 1). The study is registered with the Australia New Zealand Clinical Trials Register ACTRN12613000043730.
Figure 1.
Overview of the flow of participants through the Pre-frailty Intervention Trial.
Participants
Potential participants will be identified by clinicians working in hospital and community sections of the Division of Rehabilitation and Aged Care Services (DRACS) at Hornsby Ku-ring-gai Health Service, Sydney, Australia.
Participants who fulfil the following inclusion criteria will be invited to participate:
Man or woman aged 70 years or older;
Meet one or two CHS frailty criteria,3 and thus are considered pre-frail (table 1);
Mild or no cognitive impairment (defined as a Mini-Mental State Examination score >23).
Table 1.
Definition of Frailty Components, adapted from Cardiovascular Health Study Criteria3
| Characteristic | Criteria |
|---|---|
| Weight loss/shrinking | Self-report of ≥4.5 kg lost unintentionally in the previous 12 months or loss of ≥5% of weight in the prior year by direct measurement of weight |
| Weakness | Lowest 20% in grip strength, measured using a dynamometer (Saehen Dynamometer, model SH5001). Best of three attempts used. Men scoring 30 kg or less, women scoring 18 kg or less meet the criteria |
| Exhaustion | Answering ‘a moderate amount’ or ‘most of the time’ to either of the 2 questions from the Centre for Epidemiological Studies-Depression Scale (CES-D) indicated exhaustion: “How often did you feel that everything you did was an effort in the last week?” or “How often did you feel that you could not get going in the last week?” |
| Slowness | Time to walk 4 m, with or without a walking aid, equals 6 s or more |
| Low activity | In the past 3 months, weight bearing physical activity was not performed, more than 4 h per day were spent sitting, and went for a short walk once per month or less |
People will be ineligible to participate in the trial if they:
Live in a residential aged care facility;
Have an estimated life expectancy of less than 12 months (estimated by a score of ≤3 on a modified version of the Implicit Illness Severity Scale17);
Currently receive a treatment programme from a rehabilitation facility.
Randomisation
After consent and completion of the baseline assessment, participants will be entered into the study and randomised to intervention or control groups. Permuted block randomisation will be used,18 with a random number sequence generated by SPSS V.19 and variable block sizes of four and six randomly arranged within blocks of 10. Project personnel not otherwise involved in recruitment or data collection will manage random group allocation. The treatment allocation tables will be stored away from the research office.
Allocation concealment
The research consultant will screen for study eligibility, seek informed consent and conduct the baseline assessment. After baseline assessment is completed, the Research Consultant will telephone the central study office, and the participant will be assigned a participant number and allocated to the control or intervention group. Staff performing the outcome assessment and data analysis will be blinded to group allocation; however, owing to the nature of the trial, it is not possible to blind the participants and staff administering interventions.
Intervention
Participants assigned to the control group will receive the usual care available to older residents of Hornsby Ku-ring-gai area from their general practitioner and community services. At the study site, usual care for non-institutionalised pre-frail older people involves medical management of health conditions, allied health input, assessment of care needs and provision of care.
Participants in the intervention group will receive an interdisciplinary, multifactorial intervention for 1 year. The intervention will be individually tailored to each participant based on the following: (A) the CHS frailty characteristics present at baseline assessment; (B) additional problems identified during a detailed assessment by the physiotherapist providing the intervention programme, plus other relevant members of the interdisciplinary team; (C) ongoing reassessment by the interdisciplinary team throughout the intervention period. The assessment and intervention will be underpinned by the principles of geriatric evaluation and management.19 20 An interdisciplinary team comprising a physiotherapist, a geriatrician, a rehabilitation physician, a dietician and a nurse will deliver the intervention. All intervention staff will have experience in delivering interventions to older people. Case management and regular case conferences will assist coordination of the interdisciplinary delivery of the intervention. The treating physiotherapist will have the role as case coordinator, liaising with the participant, family, health professionals and service providers, plus coordinating services as indicated.
The intervention will be delivered primarily in participants’ homes, with additional community exercise programmes and outpatient appointments (eg, podiatrist, memory clinic, continence clinic) offered when indicated.
The interventions targeting the CHS frailty characteristics are described below.
Weight loss
A dietician will evaluate nutritional intake if the participant is not already effectively addressing their recent weight loss. If the participant's body mass index is <18.5 kg/m2 or mid-upper arm circumference <10th centile (using Australian gender-specific and age-specific norms), nutritional supplementation will be offered using commercially available, high-protein, high-energy supplements. Home delivered meals will be recommended if appropriate clinical criteria apply.
Exhaustion
Referral to a psychiatrist or psychologist will be considered if the Geriatric Depression Scale score is high. Where the participant is socially isolated, opportunities to encourage greater social engagement will be identified, for example, day activity groups, physical activity programmes in the community and telephone contact with volunteers.
Grip weakness, slow 4 m walk time or low physical activity level
A physiotherapist experienced in aged care will visit the participant's home 10 times in the 12-month study period. There will be five sessions in the first 3 months after randomisation, and five sessions over the following 9 months. Visits will be of 60–120 min duration. The physiotherapist will prescribe a home exercise programme to be performed for 20–30 min, up to six times per week, for 12 months. The exercises, degree of difficulty and number of repetitions prescribed will be based on assessment of the individual participant's abilities. Lower limb balance and strengthening exercises will utilise the Weight Bearing Exercise for Better Balance (WEBB) programme, available at http://www.webb.org.au.21 The programme targets strength and control of the lower limb extensor muscles (hip and knee extensors, ankle plantarflexors) with exercises including standing up from a chair, forward and lateral step-ups onto a block and heel raises while standing on a wedge. Resistance will be applied by body weight or by weighted vests or weight-belts as appropriate. Balance will be targeted with exercises performed while standing on a progressively narrowed base (feet together, tandem stance, single leg stance), stepping, walking and reaching. Upper limb support will be minimised in order to adequately challenge balance, but to ensure safety the environment will be set up with stable supports (eg, bench or table) close by that can be held as necessary. In addition, if upper limb weakness is creating functional problems, then the physiotherapist may prescribe upper limb exercises incorporating theraband or free weights for resistance. The physiotherapist will regularly review and modify the optimal intensity and type of exercises for each participant to ensure that the intervention remains appropriate and challenging over the study period. We will encourage family members or carers to assist with the exercise programme when this is indicated.
Appropriate safe mobility programmes will be prescribed if participants have low activity levels, reduced endurance or specific functional goals. Feedback will be provided via monitoring of distance/time or via a pedometer or FitBit (internet-linked pedometer). Participants will be encouraged and supported in increasing their physical activity using exercise equipment that they have at home, as well as community physical activity programmes (such as Tai Chi or strength and balance classes), community exercise facilities (such as gymnasiums and swimming pools) and a return to past leisure activities such as golf and bowls.
In addition to the interventions targeting the CHS frailty characteristics, individually tailored intervention will address additional problems identified during assessment. Intervention may include, but will not be limited to, the following examples.
General health status will be assessed and intervention tailored to each individual's problems. Where indicated, chronic disease management programmes will be implemented or reinforced in conjunction with existing health services. We will use the principles of comprehensive geriatric assessment, with careful follow-up of chronic diseases, pain and conditions such as incontinence, osteoporosis and impaired cognition. The rehabilitation physician and geriatrician will play a central role in assessment and recommendations for ongoing intervention.
The rehabilitation physician or geriatrician will review medications used and discuss any questionable medication use with the participant's general practitioner. Poor compliance with medications will be addressed by initiation or reinforcement of strategies such as education about medications, medication packaging in blister packs and reminder cards.
Referrals will be made as indicated to allied health, Hearing Australia, Vision Australia, and disease-specific programmes such as pulmonary rehabilitation, cardiac rehabilitation and Parkinson's disease exercise classes.
The team will refer to agencies that provide assessments and provision of care and services. Examples are the Aged Care Assessment Team for assessment of packages of care, community nursing and service providers.
If transport is required, we will arrange referral to community transport services, taxi subsidy schemes and mobility parking schemes as appropriate.
Reduced social interaction will be targeted by facilitating attendance at community-based groups, day centres, clubs and exercise groups, as well as by arranging telephone contact with a volunteer.
We will advise on meal delivery services and frozen meals if this assistance is needed.
Mobility aids and other equipment will be recommended, obtained and set up where indicated. This may involve referral to an occupational therapist for environmental modifications.
Advice on appropriate footwear will be provided if shoes are suboptimal.
Ergonomic alterations will be made to optimise home office safety.
If the participant is at risk of falling, they may be referred to falls-specific clinics (Falls and Osteoporosis Clinics) and programmes (Stepping On program, Otago Exercise Program) available in the study area, in addition to the WEBB exercise programme. Safety concerns will also be addressed with information about falls prevention, personal alarms and hip protectors.
If the participant cares for another person or the participant has a carer who needs help, the carer's needs will be assessed and contact with Carers Australia will be suggested.
The physiotherapist and participant will collaborate to set measurable goals within 3 months of recruitment. The goals will be based on the CHS frailty characteristics present (such as goals relating to diet, functional consequences of weakness or amount of physical activity), or problems identified during geriatric assessment (such as establishing formal links with a diabetes educator, understanding medications or obtaining a care package). The goals will be documented, reviewed each session by the physiotherapist and participant, and new goals will be set when new issues are targeted.
The physiotherapist will promote adherence to the intervention using strategies including goal setting, a flexible time frame for intervention delivery, recording of exercise completion, and involvement of family and carers. In addition, programmes will be tailored to suit individual requirements and interventions will be designed to be varied, sustainable and enjoyable.
Data collection
Participants will undergo three home-based assessments. The baseline measures will be assessed prior to randomisation and further assessments will be conducted 4 and 12 months after randomisation. Additional health service utilisation data will be collected via a telephone call at 8 months. Blinded assessors (experienced health professionals) will conduct follow-up assessments. To ensure blinding, participants will be instructed not to disclose group allocation to the assessors. The assessors’ perception of group allocation will be assessed to evaluate the success of assessor blinding.
Outcome measures
Demographic and health information will be collected at baseline. Cognitive function will be assessed with the Mini-Mental State Examination.22
Primary outcomes
The primary outcomes measured are frailty and mobility, measured at 4 and 12 months. Frailty will be measured using the CHS frailty phenotype3 as detailed in table 1. The frailty phenotype evaluates five components of the frailty syndrome and allocates one point for each criterion met; participants meeting zero criteria are defined as non-frail, whereas those meeting one or two criteria are defined as pre-frail, and those meeting three, four or five criteria are defined as frail. Mobility will be assessed using the lower extremity continuous summary performance score (CSPS),23 with data collected using the Short Physical Performance Battery (SPPB).24 This battery examines the ability to stand (for 10 s) with the feet together in the side-by-side, semi-tandem and tandem positions, time taken to walk 4 m, and time to rise from a chair and return to the seated position five times.
Secondary outcomes
Psychological status will be assessed using the five-item version of the Geriatric Depression Scale.25
Activities of daily living will be measured using the Barthel Index26 (100 point version). The mobility component of the Activity Measure for Post Acute Care27 will measure self-reported activity level using Item Response Theory and computer-adaptive testing.
Gait speed will be measured using the 4 m walk test.
The EQ-5D (EuroQol) will measure health-related quality of life and provide utility weights to allow calculation of quality adjusted life years (QALYs) for use in the economic evaluation.28
Falls, hospitalisations and admissions to residential aged care facilities will be collected via telephone at 4, 8 and 12 months and will also be used in the economic analyses.
Health and community service use will be recorded at 4, 8 and 12 months and will be used in economic analyses.
Additional measures
Adherence measurements will record the acceptance of health and other services by the study participant. The treating physiotherapist will estimate a global level of adherence (in five categories: 0%, <25%, 25–49%, 50–74% and ≥75%) during the 12-month intervention. The treating physiotherapist will evaluate goal attainment in the intervention group using a four-point scale: deterioration from baseline ability, maintained baseline ability, goal met, goal exceeded.
Adverse events will be defined as medical events or injuries arising as a consequence of the trial and resulting in medical attention or restricted activities of daily living for more than 2 days.29 Deaths will be documented.
Sample size calculation
An a priori power analysis determined that 230 participants will need to be recruited to provide 80% power to detect a clinically and statistically significant 15% between-group difference in the lower extremity CSPS (SD=0.7).24 This sample size will also provide sufficient power to detect a clinically meaningful 20% between-group difference in the transition to frailty. For these calculations, we assumed an α of 0.05, a non-compliance of 15% and a dropout rate of 15%.
Statistical analysis
Frailty will be treated as a dichotomous variable, scored as transitioned to frailty (ie, the number of frailty criteria was 3 or more) or did not transition to frailty (number of frailty criteria was 0, 1 or 2). The χ2 test will be used for frailty as a dichotomous variable. The other study outcomes will be treated as continuous variables. The effect of group allocation on continuously scored outcome measures at the 4-month and 12-month follow-ups will be analysed using linear regression models with baseline scores entered into the linear regression models as covariates. To aid interpretation of the change in frailty, frailty will also be reported as a continuous variable. Statistical significance will be set at p<0.05 and we will report the differences in percentage or mean (95% CI) between the two groups at the 4-month and 12-month follow-ups.
We will test whether the response to the intervention is modified by the number of frailty criteria present at baseline, by including an interaction term of study groups with the number of frailty criteria at baseline in the regression analyses.30 Secondary analyses will also explore the effect of different rates of adherence (as a category variable: <25%, 25–49%, 50–74% and ≥75%) on the outcomes in the intervention group at 12-month follow-up. We will examine baseline variables and if there are important between-group differences, we will adjust for them in the models. The primary analyses will be conducted in accordance with the intention-to-treat principle.31 Data will be coded to permit blinding to group allocation in the statistical analysis.
Participants will be provided with their own results on request. The overall results will be available to participants once the final results are published. It is anticipated that participants will register their interest in receiving this information when their participation in the study ends.
Economic evaluation
The economic evaluation will be carried out and reported in accordance with health economics reporting standards.32 The economic evaluation will take the perspective of Australian health and aged care service providers over a 12-month time period. Benefits will be measured in terms of the number of transitions to frailty prevented, mobility improvement and QALYs gained (based on utility weights derived from the EQ-5D). The cost-effectiveness analyses will include the cost of delivering the intervention and the cost of health and community service utilisation. Bootstrap sampling will be used to examine the joint probability distribution of costs and outcomes, with the creation of incremental cost-effectiveness planes and cost-effectiveness acceptability curves for each outcome.
Time frame
Recruitment started in January 2013. Follow-up assessment is expected to conclude in October 2015.
Discussion
This trial will provide important information to guide intervention to improve outcomes for older people who are pre-frail. Specifically, it will determine whether a multifactorial interdisciplinary intervention reduces transition to frailty and deterioration in mobility among pre-frail older men and women who live in the community. Frailty and the associated negative effects such as disability, institutionalisation and hospitalisation are costly to individuals, their families, the health system and society. Despite this cost, to the best of our knowledge, there has been no research to date examining the effectiveness of an intervention designed to reduce the transition to frailty among pre-frail older people.
The proposed multifactorial intervention will target the needs of each participant based on the characteristics of frailty present and comprehensive geriatric assessment. The exercise component was designed using evidence from systematic reviews and randomised trials that have demonstrated improved strength, balance and mobility in older people. We will implement strategies to maximise adherence to the intervention, in line with research suggesting that good patient adherence increases the effectiveness of health interventions.7 33 The intervention is based on the programme that was feasibly delivered to frail older people in the Frailty Intervention Trial,16 with some modifications to enable a greater challenge to balance, strength and physical activity. Tailoring the exercises to the individual and ongoing reassessment by the treating physiotherapist will ensure safety.
Additional strengths of the study are the generalisability to pre-frail older people and aged care health service settings, and the robust, but pragmatic, clinical trial design. This study uses an objective measure of pre-frailty; the CHS criteria have previously been used to recruit frail7 and pre-frail13–15 people to clinical trials. We have avoided excessive exclusion criteria. The intervention being examined is readily transferable to routine clinical practice in the aged care health service setting and the interdisciplinary approach is relevant to several professional groups in aged care.
This study has some limitations. First, participants cannot be blinded to group allocation, which is a potential source of bias due to possible differential reporting of the weight loss, activity and exhaustion frailty criteria. However, the weakness and slowness frailty criteria and the co-primary outcome measure (CSPS) are performance based, which should reduce this bias. Second, as there is no frequency-matched social intervention for the control group, we will not be able to exclude the impact of social aspects of the programme on any difference between groups. Third, there is no consensus on how to identify pre-frailty34 and while the CHS phenotype is the most widely accepted instrument, other validated tools35 and attention to cognition could be considered in the clinical setting.
If this intervention is shown to be effective, there are major potential benefits to the older population in terms of preventing transition to frailty and improving mobility. Avoiding frailty has the potential to reduce adverse health outcomes, such as fall rates, hospitalisation and institutionalisation, and the associated financial costs. Improved mobility may also result in improved function and better quality of life for older people, their families and carers. If cost-effectiveness is demonstrated, this intervention will lead to more efficient utilisation of health services. The findings will be disseminated through scientific and professional conferences and in peer-reviewed journals.
Supplementary Material
Acknowledgments
This study was supported by the Doris Whiting Special Purpose and Trust Fund, administered by the Division of Rehabilitation and Aged Care, Hornsby Ku-ring-gai Health Service, Australia.
Footnotes
Contributors: NF drafted the manuscript. CS, SRL, SEK and IDC are chief investigators on the study. NF, KL, NM, BJ and KH are actively involved in the study. All authors read and approved the final manuscript.
Funding: IDC's salary is supported by an Australian National Health and Medical Research Council Practitioner Fellowship
Competing interests: None.
Ethics approval: The Northern Sydney Local Health District Health Research Ethics Committee approved this study (Research Protocol Number 1207-213M).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morley JE, Vellas B, van Kan GA et al. . Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walston J, Hadley EC, Ferrucci L et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006;54:991–1001. 10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J et al. . Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 5.Collard RM, Boter H, Schoevers RA et al. . Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 6.Theou O, Stathokostas L, Roland KP et al. . The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011;2011:569194 10.4061/2011/569194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron ID, Fairhall N, Langron C et al. . A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med 2013;11:65 10.1186/1741-7015-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill TM, Gahbauer EA, Allore HG et al. . Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418–23. 10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- 9.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009;55:539–49. 10.1159/000211949 [DOI] [PubMed] [Google Scholar]
- 10.Faber MJ, Bosscher RJ, Chin APMJ et al. . Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil 2006;87:885–96. 10.1016/j.apmr.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Gill TM, Baker DI, Gottschalk M et al. . A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002;347:1068–74. 10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]
- 12.Gillespie LD, Robertson MC, Gillespie WJ et al. . Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9:CD007146 10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel K. Wii-hab for pre-frail older adults. Rehabil Nurs 2012;37:195–201. 10.1002/rnj.25 [DOI] [PubMed] [Google Scholar]
- 14.Lustosa LP, Silva JP, Coelho FM et al. . Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: a randomized crossover trial. Rev Bras Fisioter 2011;15:318–24. 10.1590/S1413-35552011000400010 [DOI] [PubMed] [Google Scholar]
- 15.Coelho FM, Pereira DS, Lustosa LP et al. . Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr 2012;54:415–20. 10.1016/j.archger.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 16.Fairhall N, Aggar C, Kurrle SE et al. . Frailty Intervention Trial (FIT). BMC Geriatr 2008;8:27 10.1186/1471-2318-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman J, Lurie N. Causes of increasing mortality in a nursing home population. J Am Geriatr Soc 1996;44:258–64. [DOI] [PubMed] [Google Scholar]
- 18.Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust 2002;177:565–7. [DOI] [PubMed] [Google Scholar]
- 19.Ko FC. The clinical care of frail, older adults. Clin Geriatr Med 2011;27:89–100. 10.1016/j.cger.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 20.Gallo JJ, Fulmer T, Paveza GJ et al. . Handbook of geriatric assessment. 3rd edn Frederick, MD: Aspen Publishers, 2000. [Google Scholar]
- 21.Sherrington C. Exercise which challenges balance can prevent falls in older people: meta-analysis of RCTs with meta-regression. Australian Physiotherapy Association Conference Week, Cairns, Australia, 2007. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. “A practical method for grading the cognitive state of patients for the clinician”. J Psychiatr Res 1975;12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23.Onder G, Penninx BW, Lapuerta P et al. . Change in physical performance over time in older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 2002;57:M289–93. 10.1093/gerona/57.5.M289 [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 25.Hoyl MT, Alessi CA, Harker JO et al. . Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc 1999;47:873–8. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 27.Haley SM, Coster WJ, Andres PL et al. . Score comparability of short forms and computerized adaptive testing: simulation study with the activity measure for post-acute care. Arch Phys Med Rehabil 2004;85:661–6. 10.1016/j.apmr.2003.08.097 [DOI] [PubMed] [Google Scholar]
- 28.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 29.Latham NK, Anderson CS, Lee A et al. . A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc 2003;51:291–9. 10.1046/j.1532-5415.2003.51101.x [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Lagakos SW, Ware JH et al. . Statistics in medicine-reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–94. 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 31.Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000;21:167–89. 10.1016/S0197-2456(00)00046-5 [DOI] [PubMed] [Google Scholar]
- 32.Husereau D, Drummond M, Petrou S et al. . Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231–50. 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 33.DiMatteo MR, Giordani PJ, Lepper HS et al. . Patient adherence and medical treatment outcomes: a meta-analysis. Med Care 2002;40:794–811. 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 34.Abellan van Kan G, Rolland Y, Houles M et al. . The assessment of frailty in older adults. Clin Geriatr Med 2010;26:275–86. 10.1016/j.cger.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Song X, MacKnight C et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.