Abstract
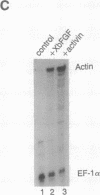
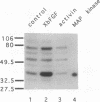
Isolated explants from the animal hemisphere of Xenopus embryos were incubated with Xenopus basic fibroblast growth factor (XbFGF) or human activin A. XbFGF incubation resulted in the rapid activation of mitogen-activated protein kinase (MAPK) and ribosomal S6 protein kinase (pp90rsk) in a dose-dependent manner with the highest levels of activation occurring at 50 ng/ml. Maximal activation occurred within 6-10 min after the addition of growth factor, and the activity of both kinases declined to unstimulated levels after 30 min. Activin was unable to activate either MAPK or pp90rsk in the Xenopus explants to a substantial level, although it induced dorsal mesoderm better than XbFGF under the same experimental conditions. The regulatory protein Xwnt-8 did not activate MAPK, nor did it enhance the activation of MAPK by XbFGF. XbFGF was able to activate MAPK through at least the midgastrula stage, suggesting that this family of growth factors may have a role in gastrula-stage events.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Krebs E. G. The mitogen-activated protein kinase activator. Curr Opin Cell Biol. 1992 Dec;4(6):992–999. doi: 10.1016/0955-0674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolce M. E., Hemmati-Brivanlou A., Kushner P. D., Harland R. M. Ventral ectoderm of Xenopus forms neural tissue, including hindbrain, in response to activin. Development. 1992 Jul;115(3):681–688. doi: 10.1242/dev.115.3.681. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Olson D. J., Moon R. T. Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J. 1992 Jan;11(1):33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Gause K. C., Homma M. K., Licciardi K. A., Seger R., Ahn N. G., Peterson M. J., Krebs E. G., Meier K. E. Effects of phorbol ester on mitogen-activated protein kinase kinase activity in wild-type and phorbol ester-resistant EL4 thymoma cells. J Biol Chem. 1993 Aug 5;268(22):16124–16129. [PubMed] [Google Scholar]
- Godsave S. F., Slack J. M. Clonal analysis of mesoderm induction in Xenopus laevis. Dev Biol. 1989 Aug;134(2):486–490. doi: 10.1016/0012-1606(89)90122-x. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Yamashita T., Hoshi M., Kawakami M., Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990 Sep 27;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kaibuchi K., Masuda T., Yamamoto T., Matsuura Y., Maeda A., Shimizu K., Takai Y. A protein factor for ras p21-dependent activation of mitogen-activated protein (MAP) kinase through MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):975–979. doi: 10.1073/pnas.90.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Maas A. Induction of dorsal and ventral mesoderm by ectopically expressed Xenopus basic fibroblast growth factor. Development. 1992 Jan;114(1):261–269. doi: 10.1242/dev.114.1.261. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Peptide growth factors and the regulation of early amphibian development. Biochim Biophys Acta. 1993 Aug 23;1155(2):227–237. doi: 10.1016/0304-419x(93)90006-x. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Varnum S. M., Wormington W. M., Melton D. A. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989 May;133(1):93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- MacNicol A. M., Muslin A. J., Williams L. T. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993 May 7;73(3):571–583. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Gotoh Y., Nishida E. Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J Biol Chem. 1993 Feb 15;268(5):3277–3281. [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993 Apr;18(4):128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993 Apr;13(4):2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. Identification in Xenopus of a structural homologue of the Drosophila gene snail. Development. 1990 Aug;109(4):967–973. doi: 10.1242/dev.109.4.967. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Jamrich M., Dawid I. B. Cell interactions and the control of gene activity during early development of Xenopus laevis. Dev Biol. 1986 Mar;114(1):238–246. doi: 10.1016/0012-1606(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Sive H. L. The frog prince-ss: a molecular formula for dorsoventral patterning in Xenopus. Genes Dev. 1993 Jan;7(1):1–12. doi: 10.1101/gad.7.1.1. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Knecht A. K., Wu M., Harland R. M. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993 Feb 11;361(6412):547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol S., Wong G. G., Melton D. A. A mouse macrophage factor induces head structures and organizes a body axis in Xenopus. Science. 1990 Aug 3;249(4968):561–564. doi: 10.1126/science.2382134. [DOI] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Involvement of p21ras in Xenopus mesoderm induction. Nature. 1992 May 21;357(6375):252–254. doi: 10.1038/357252a0. [DOI] [PubMed] [Google Scholar]