Abstract
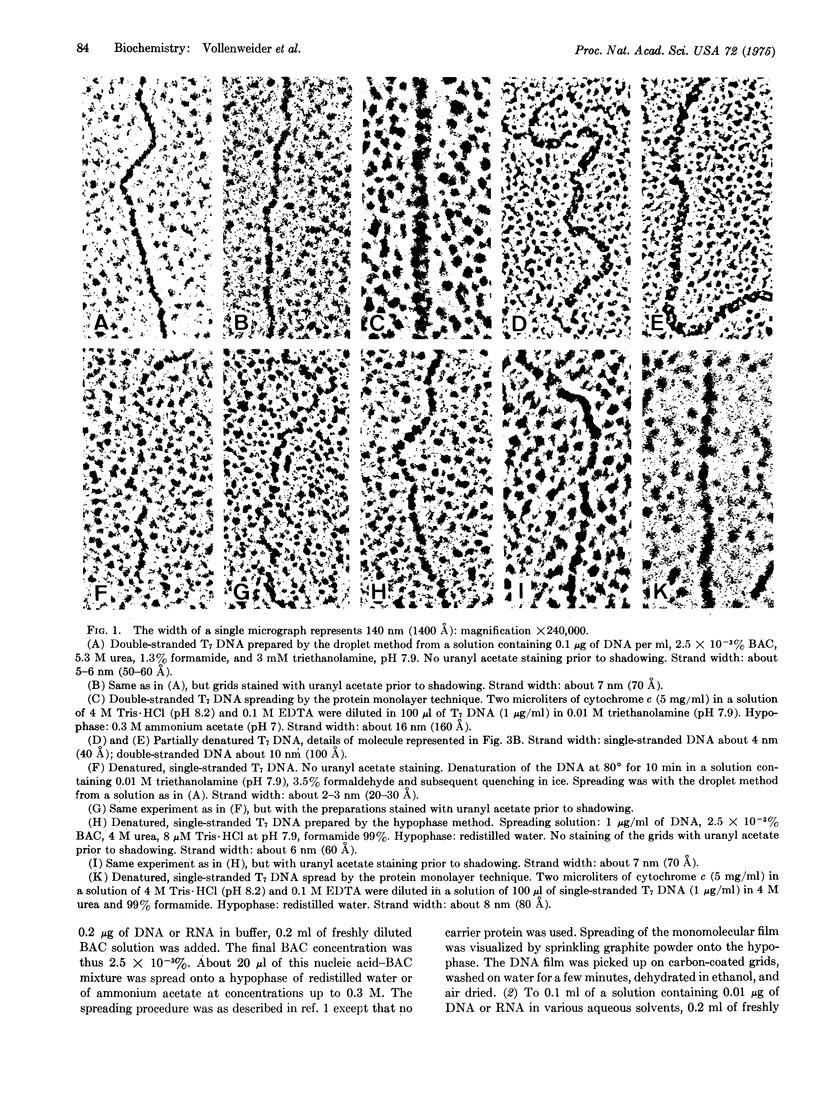
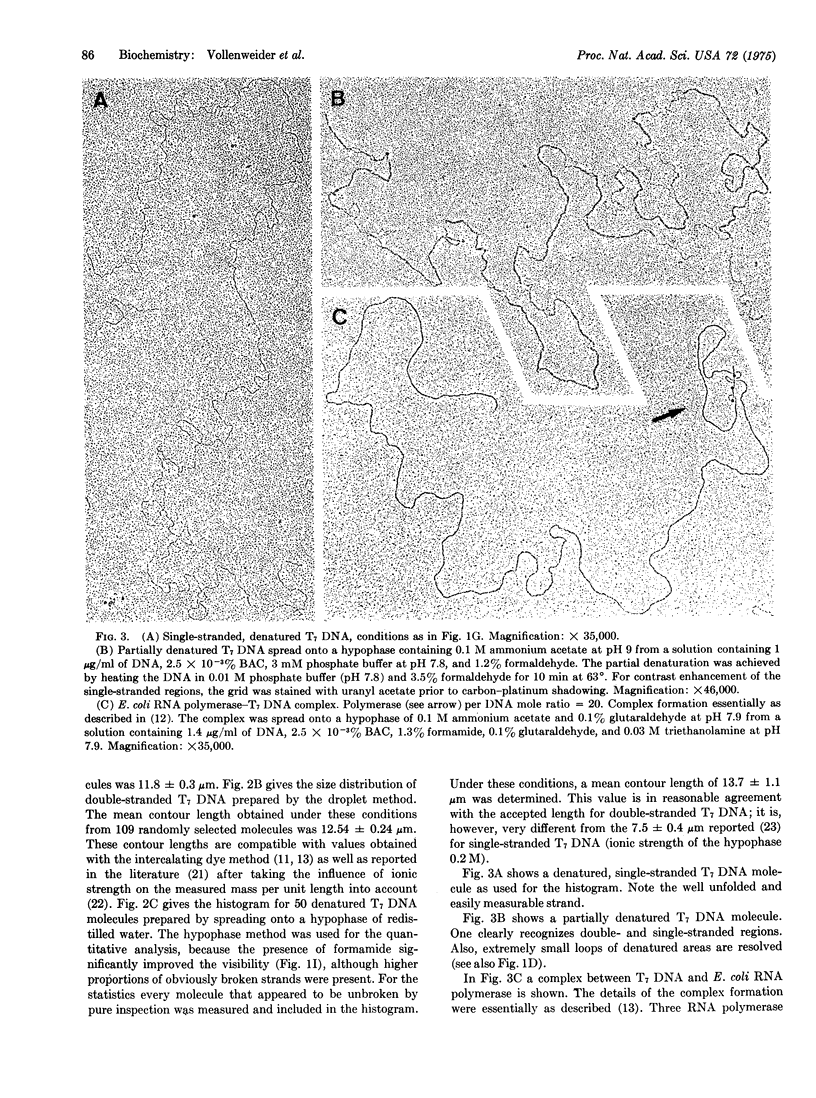
A protein-free nucleic acid preparation method for electron microscopy is described. The basic procedure is very similar to the classical protein monolayer spreading techniques. The carrier protein (usually cytochrome c) is replaced by benzyldimethylalkylammonium chloride. Both the hypophase method and the microdiffusion or droplet method can be applied with this compound. Unlike cytochrome c, benzyldimethylalkylammonium chloride does not lead to any apparent thickening of the nucleic acid strands. Partially denatured DNA spread with this reagent shows a loosened structure with a foamy appearance in the regions previously considered to be "unmelted," which open up locally into melted loops of different size. Specifically bound proteins, such as RNA polymerase on bacteriophage T7 DNA, can be detected unambiguously.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER M., MOUDRIANAKISEN Determination of base sequence in nucleic acids with the electron microscope: visibility of a marker. Proc Natl Acad Sci U S A. 1962 Mar 15;48:409–416. doi: 10.1073/pnas.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Dubochet J. Electron microscopic localization of the binding sites of Escherichia coli RNA polymerase in the early promoter region of T7 DNA. Eur J Biochem. 1974 May 15;44(2):617–624. doi: 10.1111/j.1432-1033.1974.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Bujard H. Electron microscopy of single-stranded DNA. J Mol Biol. 1970 Apr 14;49(1):125–137. doi: 10.1016/0022-2836(70)90381-5. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Hamkalo B. A., Miller O. L., Jr Electronmicroscopy of genetic activity. Annu Rev Biochem. 1973;42:379–396. doi: 10.1146/annurev.bi.42.070173.002115. [DOI] [PubMed] [Google Scholar]
- Harford A. G., Beer M. Electron-microscopic localization of the binding of Escherichia coli RNA polymease to T7 DNA in vitro. J Mol Biol. 1972 Aug 21;69(2):179–186. doi: 10.1016/0022-2836(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Portmann R., Sogo J. M., Koller T., Zillig W. Binding sites of E. coli RNA polymerase on T7 DNA as determined by electron microscopy. FEBS Lett. 1974 Sep 1;45(1):64–67. doi: 10.1016/0014-5793(74)80811-2. [DOI] [PubMed] [Google Scholar]
- Thach S. S., Thach R. E. Mechanism of viral replication. I. Structure of replication complexes of R17 bacteriophage. J Mol Biol. 1973 Dec 15;81(3):367–380. doi: 10.1016/0022-2836(73)90147-2. [DOI] [PubMed] [Google Scholar]
- Wartell R. M. The helix-coil transitions of poly (dA)-poly (dT) and poly (dA-dT)-poly (dA-dT). Biopolymers. 1972;11(4):745–759. doi: 10.1002/bip.1972.360110403. [DOI] [PubMed] [Google Scholar]




