Abstract
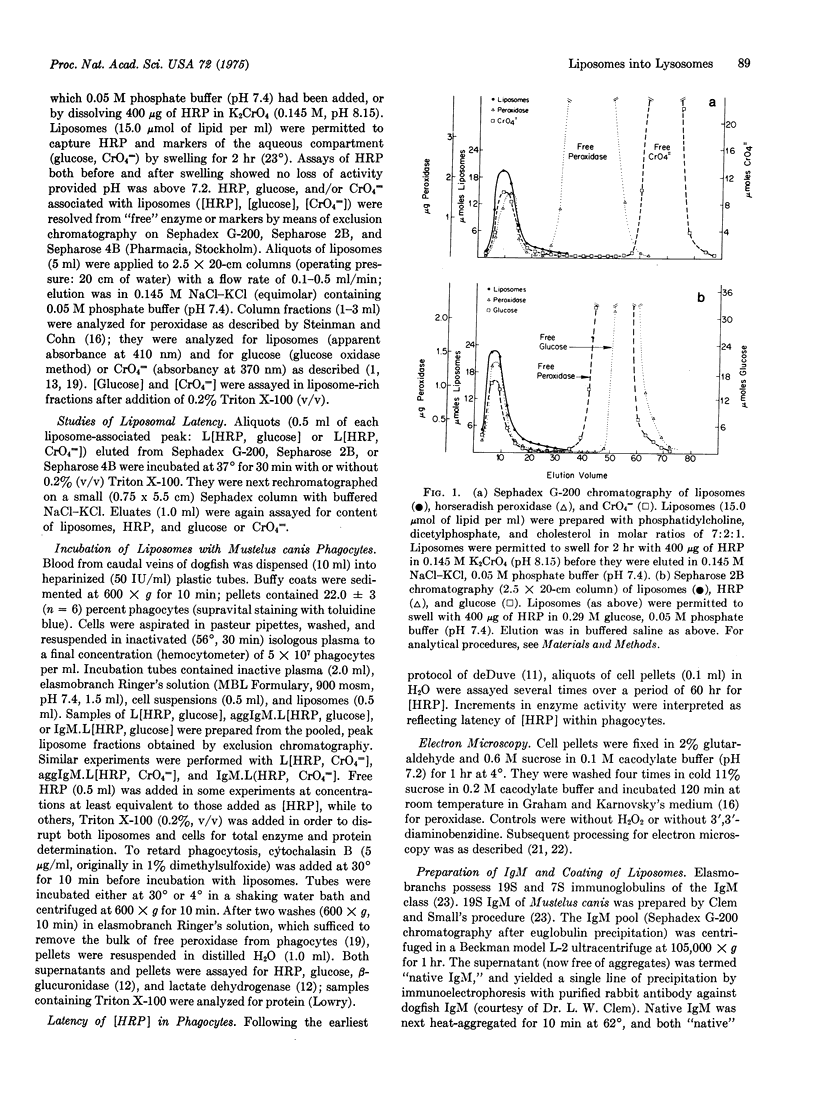
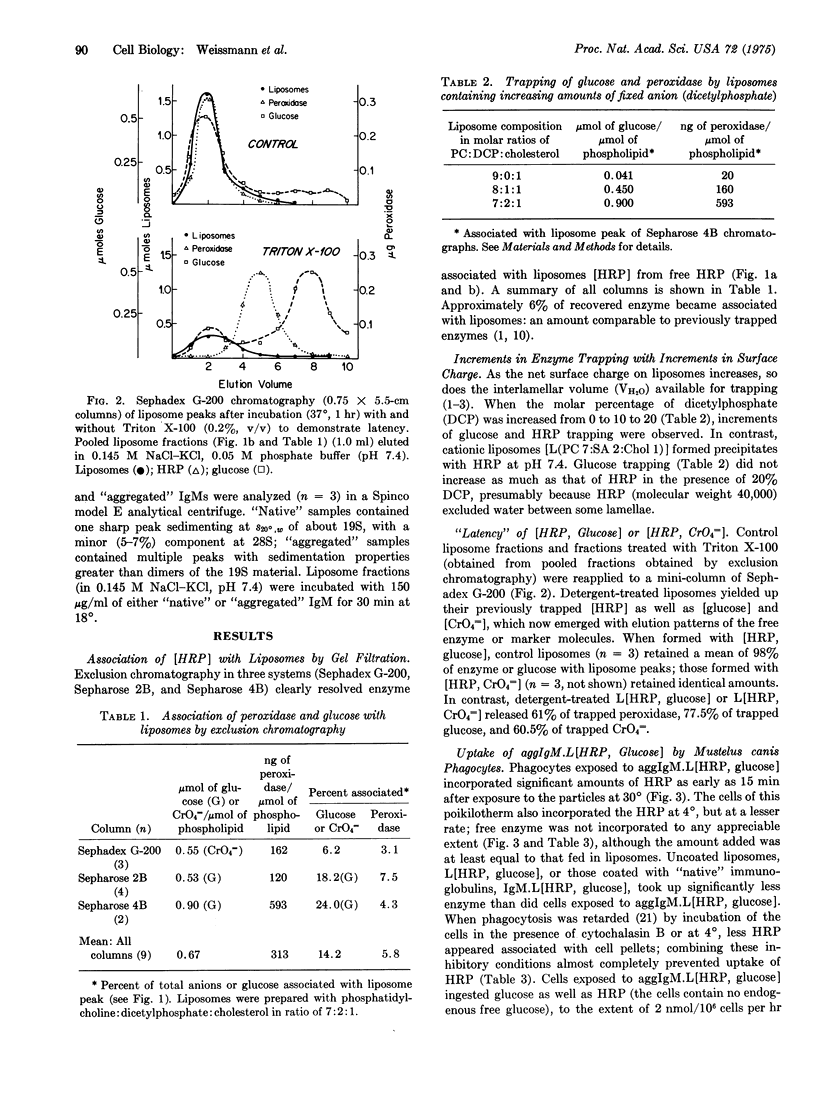
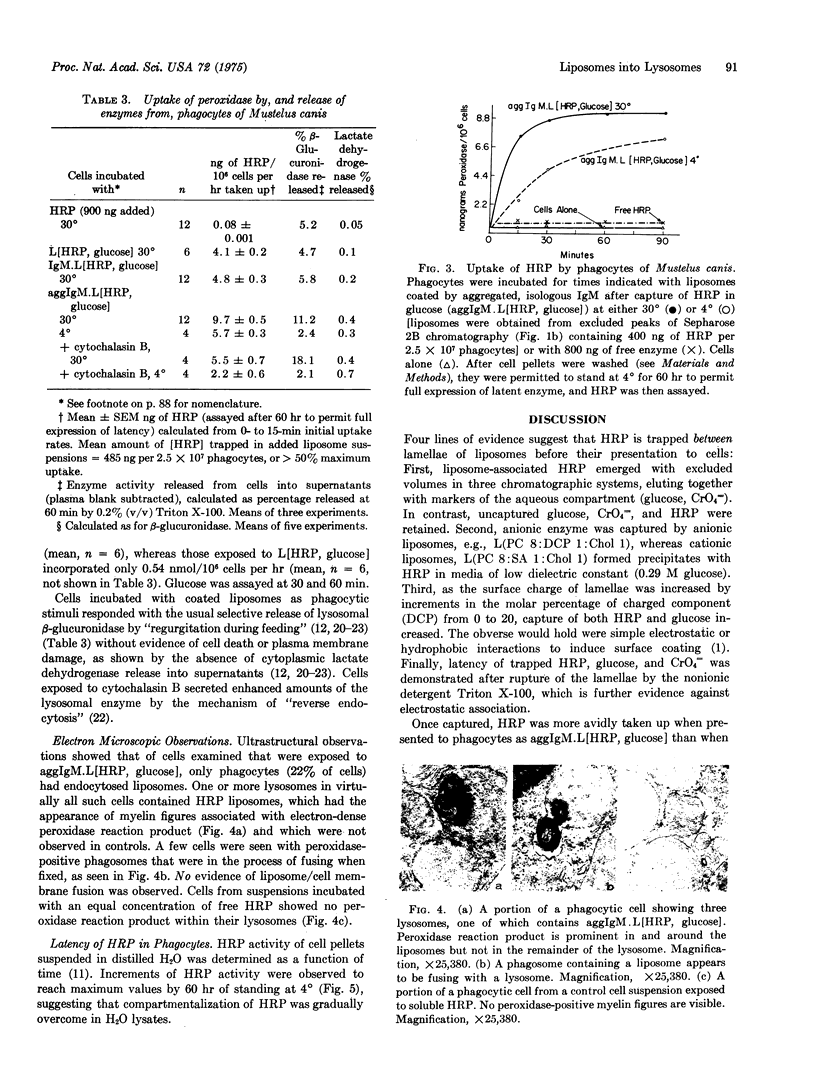
Phagocytes of the smooth dogfish (Mustelus canis) contain no endogenous peroxidase within their lysosomes and constitute models for cells genetically deficient in lysosomal enzymes such as myeloperoxidase. We have obtained uptake of over 50% of exogenous horseradish peroxidase, provided the enzyme is exhibited to cells after incorporation into liposomes coated with heat-aggregated (62 degrees, 10 min), isologous IgM. Trapping of horseradish peroxidase (EC 1.11.1.7) by liposomes was established by chromatographic resolution (Sephadex G-200; Sepharose 2B and 4B) of free enzyme from that associated with liposomes; liposome-associated horseradish peroxidase, together with trapped markers of the aqueous compartment (glucose, CrO4 equals), were excluded, and free enzyme and markers were retained. Enzyme and marker trapping was not electrostatic, varied with the molar ratio of charged membrane components, and was reversed by detergent lysis (Triton X-100) of liposomes. Uptake at 30 degrees of aggregated IgM-coated liposomes containing trapped horseradish peroxidase exceeded that of free enzyme of 100-fold, and was more efficient than uptake of horseradish peroxidase presented in uncoated liposomes or in liposomes coated with native IgM. After phagocytosis, peroxidase-rich liposomes were localized exclusively in lysosomes of the phagocytes by ultrastructural histochemistry; the enzyme displayed over 50% latency to osmotic lysis. This method may prove to be of general use in the provision of exogenous enzymes to phagocytic cells genetically deficient in lysosomal hydrolases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clem L. W., Small P. A., Jr Phylogeny of immunoglobulin structure and function. I. Immunoglobulins of the lemon shark. J Exp Med. 1967 May 1;125(5):893–920. doi: 10.1084/jem.125.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases. Eur J Biochem. 1972 Jan 21;24(3):485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Liposomes containing antiviral antibody can protect cells from virus infection. Nature. 1972 Feb 11;235(5337):339–341. doi: 10.1038/235339a0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Huang L., Wey C. Interaction of phospholipid vesicles with cultured mammalian cells. Nature. 1974 Nov 8;252(5479):166–167. doi: 10.1038/252166a0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E. Fusion of mammalian cells by unilamellar lipid vesicles: inflluence of lipid surface charge, fluidity and cholesterol. Biochim Biophys Acta. 1973 Sep 27;323(1):23–42. doi: 10.1016/0005-2736(73)90429-x. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Patel B. C. Binding of a solubilized membrane ATPase to phospholipid bilayers. Biochim Biophys Acta. 1974 Aug 21;363(1):70–85. doi: 10.1016/0005-2736(74)90007-8. [DOI] [PubMed] [Google Scholar]
- Sessa G., Weissmann G. Incorporation of lysozyme into liposomes. A model for structure-linked latency. J Biol Chem. 1970 Jul 10;245(13):3295–3301. [PubMed] [Google Scholar]
- Sessa G., Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res. 1968 May;9(3):310–318. [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Brand A., Franklin E. C. Interaction of immunoglobulins with liposomes. J Clin Invest. 1974 Feb;53(2):536–543. doi: 10.1172/JCI107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Rita G. A. Molecular basis of gouty inflammation: interaction of monosodium urate crystals with lysosomes and liposomes. Nat New Biol. 1972 Dec 6;240(101):167–172. doi: 10.1038/newbio240167a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J Cell Biol. 1973 Jul;58(1):27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]



