Abstract
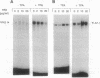
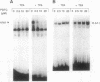
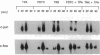
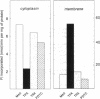
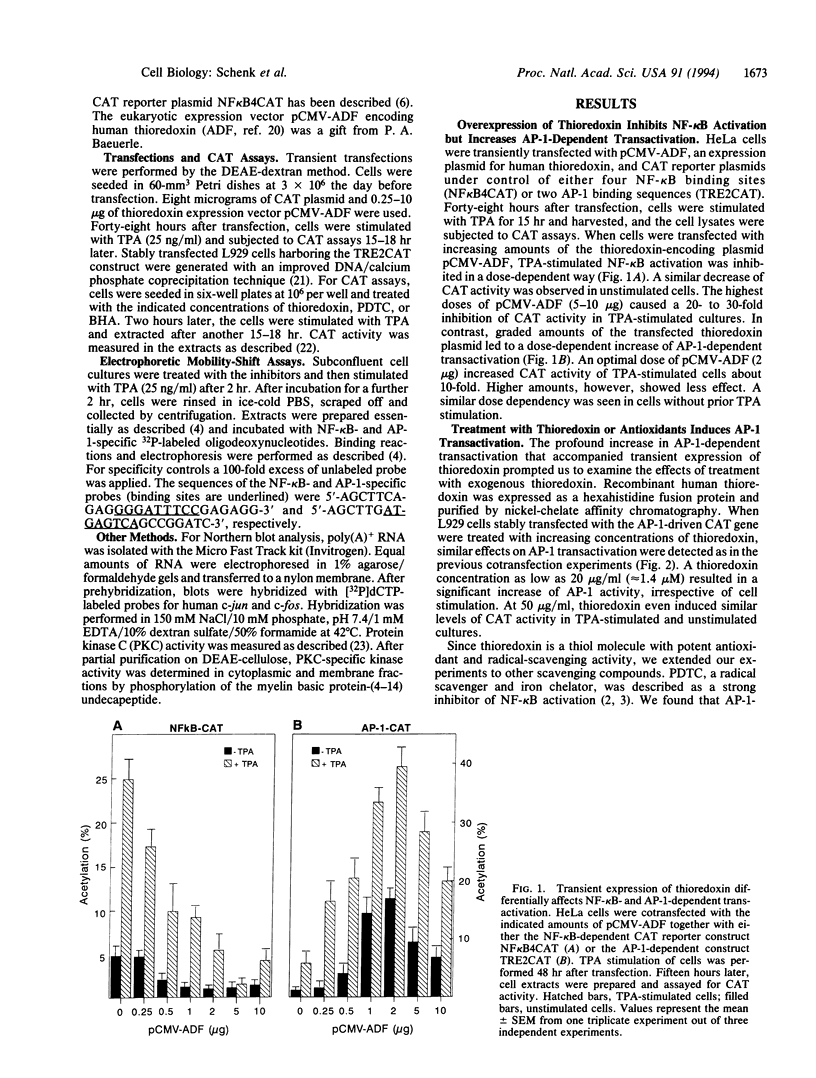
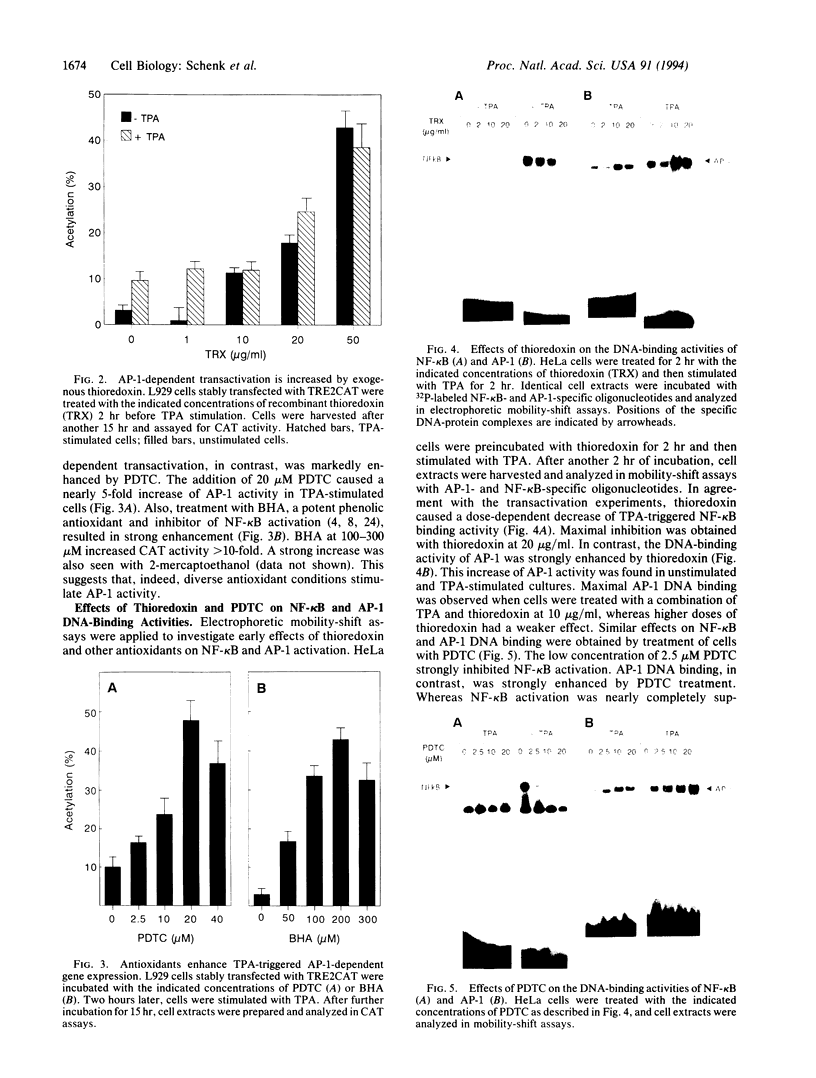
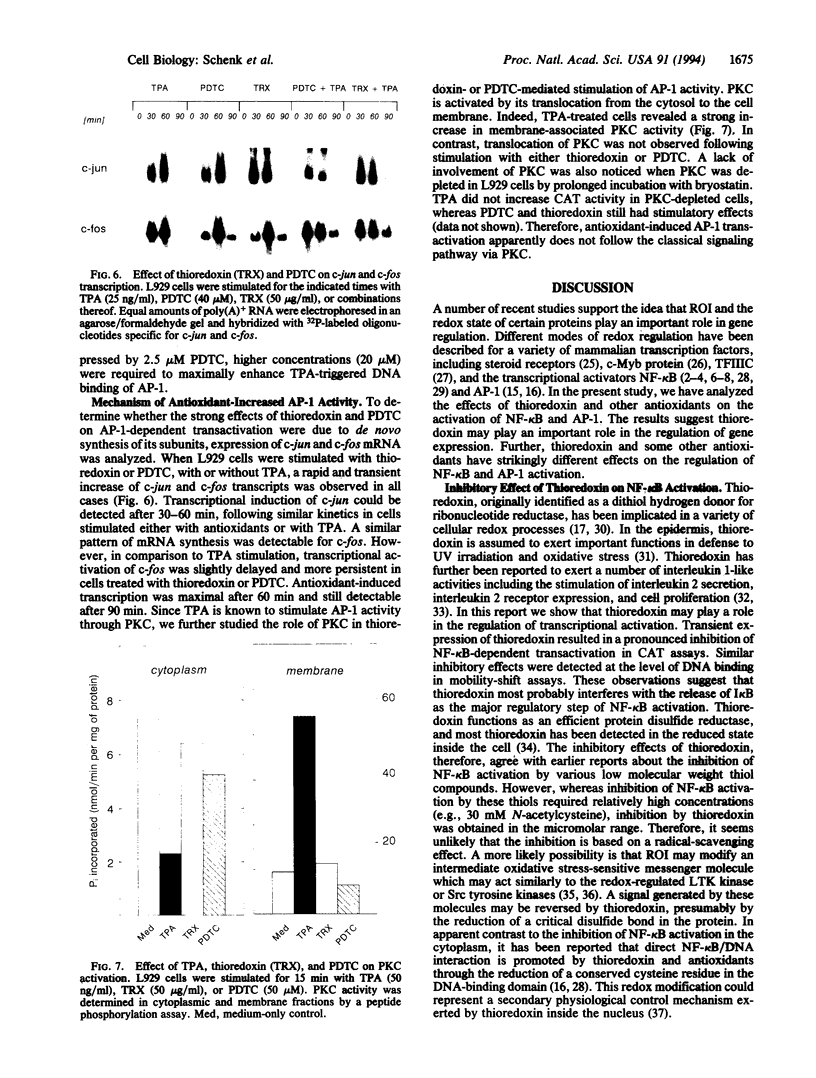
The transcription factors NF-kappa B and AP-1 have been implicated in the inducible expression of a variety of genes involved in responses to oxidative stress and cellular defense mechanisms. Here, we report that thioredoxin, an important cellular protein oxidoreductase with antioxidant activity, exerts different effects on the activation of NF-kappa B and AP-1. Transient expression or exogenous application of thioredoxin resulted in a dose-dependent inhibition of NF-kappa B activity, as demonstrated in gel shift and transactivation experiments. AP-1-dependent transactivation, in contrast was strongly enhanced by thioredoxin. A similar increase of AP-1 activity was also observed with other, structurally unrelated antioxidants such as pyrrolidine dithiocarbamate and butylated hydroxyanisole, indicating that the thioredoxin-induced increase of AP-1 activation was indeed based on an antioxidant effect. Moreover, the stimulatory effect on AP-1 activity was found to involve de novo transcription of the c-jun and c-fos components but to be independent of protein kinase C activation. These results suggest that thioredoxin plays an important role in the regulation of transcriptional processes and oppositely affects NF-kappa B and AP-1 activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Cromlish J. A., Roeder R. G. Human transcription factor IIIC (TFIIIC). Purification, polypeptide structure, and the involvement of thiol groups in specific DNA binding. J Biol Chem. 1989 Oct 25;264(30):18100–18109. [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Farrar Y. J., Vanaman T. C., Slevin J. T. A phosphatase resistant substrate for the assay of protein kinase C in crude tissue extracts. Biochem Biophys Res Commun. 1991 Oct 31;180(2):694–701. doi: 10.1016/s0006-291x(05)81121-0. [DOI] [PubMed] [Google Scholar]
- Fernando M. R., Nanri H., Yoshitake S., Nagata-Kuno K., Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992 Nov 1;209(3):917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehmann S., Vorbrueggen G., Kalkbrenner F., Moelling K. Reduction of a conserved Cys is essential for Myb DNA-binding. Nucleic Acids Res. 1992 May 11;20(9):2279–2286. doi: 10.1093/nar/20.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ueno Y., Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993 May 25;268(15):11380–11388. [PubMed] [Google Scholar]
- Herrlich P., Ponta H., Rahmsdorf H. J. DNA damage-induced gene expression: signal transduction and relation to growth factor signaling. Rev Physiol Biochem Pharmacol. 1992;119:187–223. doi: 10.1007/3540551921_7. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Israël N., Gougerot-Pocidalo M. A., Aillet F., Virelizier J. L. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992 Nov 15;149(10):3386–3393. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm S., Ennen J., Pessara U., Kurth R., Dröge W. Inhibition of HIV-1 replication and NF-kappa B activity by cysteine and cysteine derivatives. AIDS. 1991 May;5(5):497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Nose K., Shibanuma M., Kikuchi K., Kageyama H., Sakiyama S., Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991 Oct 1;201(1):99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Ogiwara H., Hayashi T., Mitsui A., Kawabe T., Yodoi J. Human thioredoxin/adult T cell leukemia-derived factor activates the enhancer binding protein of human immunodeficiency virus type 1 by thiol redox control mechanism. Int Immunol. 1992 Jul;4(7):811–819. doi: 10.1093/intimm/4.7.811. [DOI] [PubMed] [Google Scholar]
- Radler-Pohl A., Sachsenmaier C., Gebel S., Auer H. P., Bruder J. T., Rapp U., Angel P., Rahmsdorf H. J., Herrlich P. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 1993 Mar;12(3):1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter K. U., Wood J. M. Free radical reduction in the human epidermis. Free Radic Biol Med. 1989;6(5):519–532. doi: 10.1016/0891-5849(89)90045-2. [DOI] [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Myers D. E., Ledbetter J. A., Uckun F. M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993 Aug 15;82(4):1212–1220. [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. M., Cidlowski J. A. Direct evidence for intra- and intermolecular disulfide bond formation in the human glucocorticoid receptor. Inhibition of DNA binding and identification of a new receptor-associated protein. J Biol Chem. 1989 Apr 25;264(12):6638–6647. [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y., Maeda Y., Mitsui A., Kondo N., Matsui H., Hamuro J., Brown N., Arai K., Yokota T., Wakasugi H. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989 Mar;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y., Wakasugi H., Masutani H., Nakamura H., Iwata S., Mitsui A., Fujii S., Wakasugi N., Tursz T., Yodoi J. Role of ATL-derived factor (ADF) in the normal and abnormal cellular activation: involvement of dithiol related reduction. Mol Immunol. 1990 Dec;27(12):1279–1289. doi: 10.1016/0161-5890(90)90032-u. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi H., Rimsky L., Mahe Y., Kamel A. M., Fradelizi D., Tursz T., Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci U S A. 1987 Feb;84(3):804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T. Diseases associated with HTLV-I: virus, IL-2 receptor dysregulation and redox regulation. Immunol Today. 1992 Oct;13(10):405–411. doi: 10.1016/0167-5699(92)90091-K. [DOI] [PubMed] [Google Scholar]