Abstract
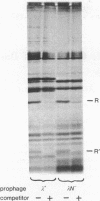
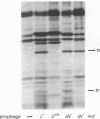
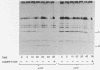
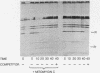
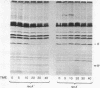
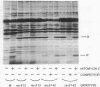
The bacteriophage lambda repressor, a protein that maintains the lysogenic state of a bacterium containing a lambda prophage, is cleaved when the lysogen is induced by mitomycin C or ultraviolet light. This cleavage does not occur when induction is prevented by mutational alteration either of the phage repressor or of the host recA gene product. Proteolytic cleavage may be the primary mechanism of repressor inactivation in this induction pathway, or it may follow a different event which causes the initial inactivation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek E., Ryan A. Lysogenic induction. Prog Nucleic Acid Res Mol Biol. 1973;13:249–300. doi: 10.1016/s0079-6603(08)60105-1. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Ebner E., Mason T. L., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. II. Effect of nuclear and extrachromosomal mutations on the formation of cytochrome c oxidase. J Biol Chem. 1973 Aug 10;248(15):5369–5378. [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noack D., Klaus S. Inactivation kinetics of lambda phage repressors in a mutant of E. coli temperature sensitive in DNA replication. Mol Gen Genet. 1972;115(3):216–224. doi: 10.1007/BF00268885. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Ito T. Inactivation of DNA-binding activity of repressor in extracts of lambda-lysogen treated with mitomycin C. Mol Gen Genet. 1973 Nov 2;126(2):103–110. doi: 10.1007/BF00330987. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Effect of ultraviolet irradiation on bacteriophage lambda immunity. J Mol Biol. 1967 Jan 28;23(2):247–263. doi: 10.1016/s0022-2836(67)80031-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]