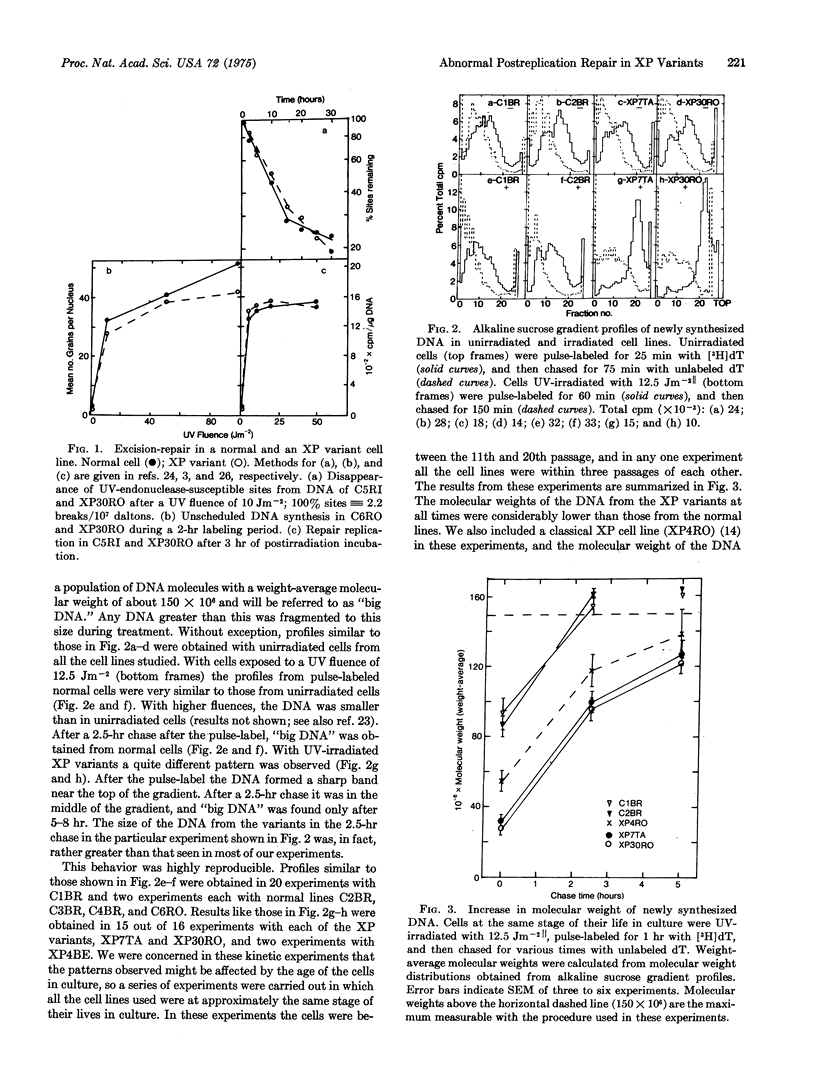
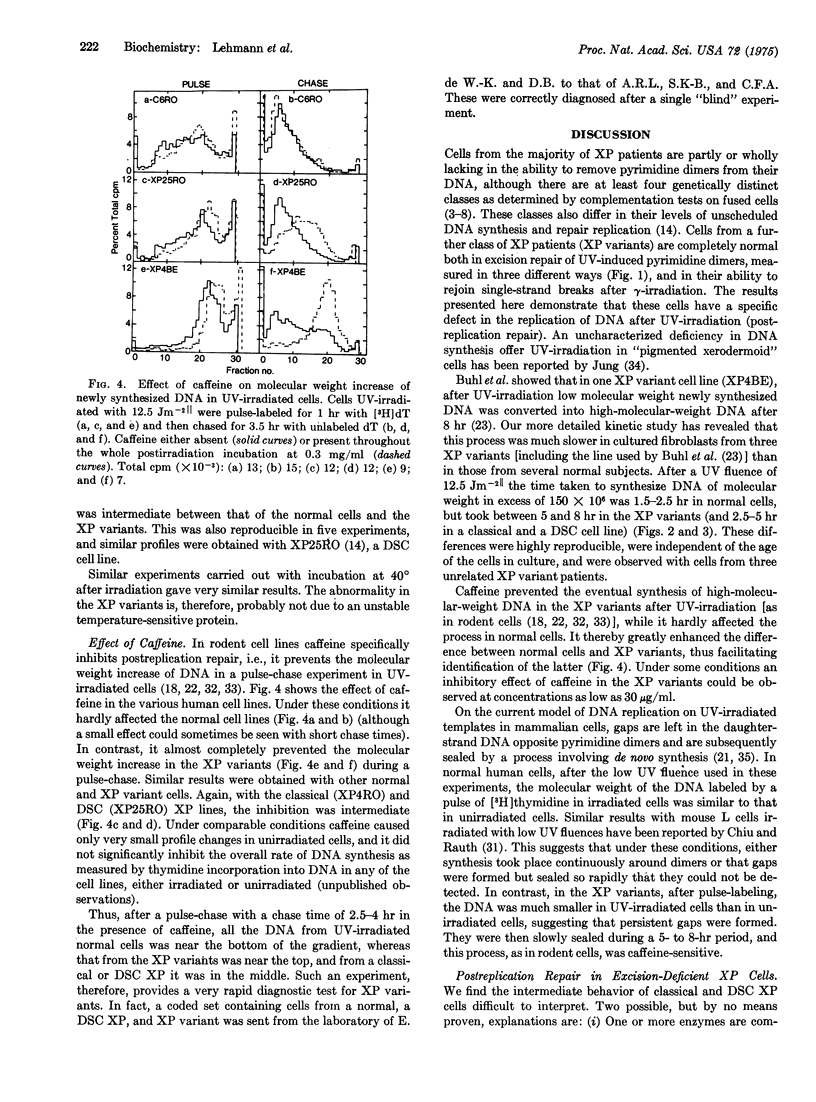
Abstract
Cells cultured from most patients suffering from the sunlight-sensitive hereditary disorder xeroderma pigmentosum are defective in the ability to excise ultraviolet light (UV)-induced pyrimidine dimers from their DNA. There is, however, one class of these patients whose cells are completely normal in this excision repair process. We have found that these cells have an abnormality in the manner in which DNA is synthesized after UV-irradiation. The time taken to convert initially low-molecular-weight DNA synthesized in UV-irradiated cells into high-molecular-weight DNA similar in size to that in untreated cells is much greater in these variants than in normal cells. Furthermore, this slow conversion of low to high-molecular-weight newly synthesized DNA is drastically inhibited by caffeine, which has no effect in normal cells. Two cell lines from classes of xeroderma pigmentosum that are defective in excision-repair show intermediate effects, with regard to both the time taken to convert newly synthesized DNA to high molecular weight and the inhibition of this process by caffeine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bootsma D., Mulder M. P., Cohen J. A., Pot F. Different inherited levels of DNA repair replication in xeroderma pigmentosum cell strains after exposure to ultraviolet irradiation. Mutat Res. 1970 May;9(5):507–516. doi: 10.1016/0027-5107(70)90035-7. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Regan J. D. Effect of caffeine on postreplication repair in human cells. Biophys J. 1974 Jul;14(7):519–527. doi: 10.1016/S0006-3495(74)85932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Stillman R. M., Setlow R. B., Regan J. D. DNA chain elongation and joining in normal human and xeroderma pigmentosum cells after ultraviolet irradiation. Biophys J. 1972 Sep;12(9):1183–1191. doi: 10.1016/S0006-3495(72)86154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk P. G., Lutzner M. A., Clarke D. D., Robbins J. H. Ultraviolet-stimulated thymidine incorporation in xeroderma pigmentosum lymphocytes. J Lab Clin Med. 1971 May;77(5):759–767. [PubMed] [Google Scholar]
- Burk P. G., Yuspa S. H., Lutzner M. A., Robbins J. H. Xeroderma pigmentosum and D. N. A. repair. Lancet. 1971 Mar 20;1(7699):601–601. doi: 10.1016/s0140-6736(71)91203-7. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Chiu S. F., Rauth A. M. Nascent DNA synthesis in ultraviolet light-irradiated mouse L cells. Biochim Biophys Acta. 1972 Jan 31;259(2):164–174. doi: 10.1016/0005-2787(72)90056-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and radiation sensitivity in human (xeroderma pigmentosum) cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(6):557–565. doi: 10.1080/09553007014551491. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Sedimentation of DNA from human fibroblasts irradiated with ultraviolet light: possible detection of excision breaks in normal and repair-deficient xeroderma pigmentosum cells. Radiat Res. 1974 Feb;57(2):207–227. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972 Mar;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Studies on repair of adenovirus 2 by human fibroblasts using normal, xeroderma pigmentosum, and xeroderma pigmentosum heterozygous strains. Cancer Res. 1974 Aug;34(8):1965–1970. [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972 Jul 19;238(81):80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- Domon M., Barton B., Porte A., Rauth A. M. The interaction of caffeine with ultra-violet-light-irradiated DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):395–399. doi: 10.1080/09553007014550481. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Characteristics of DNA synthesis following ultraviolet light irradiation in mouse L cells. Postreplication repair. Exp Cell Res. 1972 Dec;75(2):485–489. doi: 10.1016/0014-4827(72)90456-9. [DOI] [PubMed] [Google Scholar]
- Harm W. Analysis of photoenzymatic repair of UV lesions in DNA by single light flashes. 8. Inhibition of photoenzymatic repair of UV lesions in E. coli DNA by caffeine. Mutat Res. 1970 Oct;10(4):319–333. doi: 10.1016/0027-5107(70)90046-1. [DOI] [PubMed] [Google Scholar]
- Jung E. G. New form of molecular defect in xeroderma pigmentosum. Nature. 1970 Oct 24;228(5269):361–362. doi: 10.1038/228361a0. [DOI] [PubMed] [Google Scholar]
- Kleijer W. J., de Weerd-Kastelein E. A., Sluyter M. L., Keijzer W., de Wit J., Bootsma D. UV-induced DNA repair synthesis in cells of patients with different forms of xeroderma pigmentosum and of heterozygotes. Mutat Res. 1973 Dec;20(3):417–428. doi: 10.1016/0027-5107(73)90062-6. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S. Effects of caffeine and theophylline on DNA synthesis in unirradiated and UV-irradiated mammalian cells. Mutat Res. 1974 Apr;26(2):73–82. doi: 10.1016/s0027-5107(74)80037-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Ormerod M. G. Artefact in the measurement of the molecular weight of pulse labelled DNA. Nature. 1969 Mar 15;221(5185):1053–1056. doi: 10.1038/2211053b0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Lohman P. H., Sluyter M. L., Matthijs I. A., Kleijer W. J. Repair replication in human cells studied by sodium iodide isopycnic centrifugation of DNA in a fixed-angle rotor. Anal Biochem. 1973 Jul;54(1):178–187. doi: 10.1016/0003-2697(73)90261-3. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Westerveld A., Sluyter M. L. DNA repair monitored by an enzymatic assay in multinucleate xeroderma pigmentosum cells after fusion. Nature. 1974 Mar 1;248(5443):50–52. doi: 10.1038/248050a0. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Burk P. G. Relationship of DNA repair to carcinogenesis in xeroderma pigmentosum. Cancer Res. 1973 May;33(5):929–935. [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Levis W. R., Miller A. E. Xeroderma pigmentosum epidermal cells with normal UV-induced thymidine incorporation. J Invest Dermatol. 1972 Nov;59(5):402–408. doi: 10.1111/1523-1747.ep12627528. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Chu E. H. Inhibition of repair of UV-damaged DNA by caffeine and mutation induction in Chinese hamster cells. Chem Biol Interact. 1973 May;6(5):317–332. doi: 10.1016/0009-2797(73)90046-x. [DOI] [PubMed] [Google Scholar]
- de Weerd-Kastelein E. A., Keijzer W., Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974 Jan;22(1):87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]
- de Weerd-Kastelein E. A., Kleijer W. J., Sluyter M. L., Keijzer W. Repair replication in heterokaryons deprived from different repair-deficient xeroderma pigmentosum strains. Mutat Res. 1973 Aug;19(2):237–243. doi: 10.1016/0027-5107(73)90082-1. [DOI] [PubMed] [Google Scholar]


