Abstract
Background
Our aim was to describe the demographics, risk factors, clinical signs, severity, and outcome of ocular surface disease (OSD) in 12 patients who had undergone trabeculectomy augmented with mitomycin C (MMC).
Methods
Twelve glaucoma patients were referred to the Dry Eye Clinic (Singapore National Eye Centre) for further management of clinically significant OSD.
Results
Of the 15 eyes from 12 patients, 14 were treated with MMC and one with 5-fluorouracil. Mean age was 69.3±10.6 years and two-thirds were male. The median interval before onset of dry eye symptoms after surgery was 13.5 months. Mean tear breakup time (TBUT) was 5.32 seconds and mean Schirmer score was 6.14 mm/5 min. Possible major risk factors for OSD in the cases include limbal stem cell deficiency occurring from exposure to antimetabolites, chronic use of antiglaucoma medications prior to surgery, and the preoperative status of the ocular surface prior to disease onset. Treatment of OSD resulted in improved best corrected visual acuity (BCVA) in 50% of the patients, with a median gain of two-line improvement in BCVA.
Conclusion
OSD is a clinical problem often overlooked in patients who undergo antimetabolite-augmented filtration surgery. Recognition of the condition and appropriate treatment can improve patient symptoms and reduce health-care burdens on the economy.
Keywords: cornea, glaucoma, ocular surface, trabeculectomy
Video abstract
Background
Dry eye disease, or ocular surface disease (OSD), as defined by the International Dry Eye Workshop (2007),1 is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface. It is associated with an increase in tear osmolarity and inflammation of the ocular surface.1 In clinical practice, an eye is considered to have OSD if there are symptoms of ocular discomfort such as gritty sensation, burning or transient blurring of vision, accompanied by objective evidence of dryness, as evidenced by a reduced tear breakup time (TBUT) or a lower Schirmer score without topical anesthesia.
Trabeculectomy is the surgical treatment of choice to lower the intraocular pressure in eyes that are suboptimally controlled with topical antiglaucoma medications alone. Although the success rate of trabeculectomy has significantly improved with the standard use of mitomycin C (MMC) and other adjunctive antimetabolite treatment,2 complications such as OSD may compromise visual function postoperatively. These concerns are important because, together, glaucoma and OSD may greatly increase the health-care burdens of the economy.3
OSD is often associated with Sjogren’s syndrome and other ocular disorders such as blepharitis and meibomian gland dysfunction (MGD). There have been several reports on the development of OSD after refractive surgery, specifically photorefractive keratectomy.4,5 However, no known studies have described the outcomes of dry eye disease that occur after trabeculectomy with adjunctive use of MMC.
In this study, we report a case series of OSD following trabeculectomy in 12 patients at our institution.
Methods
Twelve glaucoma patients were referred to the Dry Eye Clinic from the Glaucoma Service after developing persistent symptoms and signs of dry eye disease. An eye was considered to have OSD if patients experienced ocular discomfort such as gritty sensation, burning or transient blurring of vision, and an associated abnormal TBUT value or Schirmer score (without anesthesia).
Each patient was reviewed by the attending ophthalmologist with a special interest in dry eye disorders. The patients were queried about their symptoms and examined accordingly. Examination of the eyes was performed, with particular attention directed toward identifying signs of OSD, which included the presence of conjunctival injection, fluorescein staining of the cornea, and presence of meibomian gland dysfunction (MGD).
A detailed profile of each patient’s history of glaucoma was also documented. The demographic data included sex, age, and ethnicity. Further information regarding the type of glaucoma, the different types of antiglaucoma medications each patient received before surgery, and the time of onset of OSD following trabeculectomy was also recorded.
Results
Demographics
The average age was 69.3±10.6 years, and among these, 66.7% (8/12) were men and 33.3% (4/12) were women. There were 10 Chinese patients (83.3%) and two Asian Indians (16.7%).
Glaucoma history and management
Of the 15 eyes that underwent trabeculectomy surgery, 14 had MMC and one had 5-fluorouracil. Six eyes (40%) had a diagnosis of primary open-angle glaucoma, seven eyes (46.7%) had primary angle-closure glaucoma, and two eyes (13%) had glaucoma due to secondary causes (Table 1).
Table 1.
Etiology of glaucoma in eyes treated with trabeculectomy/MMC (n=15)
| Etiology of glaucoma | % (n) |
|---|---|
| Primary | |
| POAG | 40.0 (6) |
| PACG | 46.7 (7) |
| Secondary | |
| Aphakic eye | 6.7 (1) |
| Stevens–Johnson syndrome | 6.7 (1) |
Abbreviations: MMC, mitomycin C; POAG, primary open-angle glaucoma; PACG, primary angle-closure glaucoma.
Topical eyedrops used to treat glaucoma in these patients prior to surgery varied (Figure 1). Timolol maleate, a beta-adrenergic blocker, was the most common intraocular pressure-lowering topical eyedrop used in these patients, with 11 out of 12 patients previously or currently on this medication. Prostaglandin analogs followed, with seven patients (58.3%) on Travatan® and three patients (25%) on Xalatan® eyedrops. Alphagan® was used by six patients (50%). Carbonic anhydrase inhibitors, such as Brinzolamide® and Dorzolamide®, were noted to be used in 16.6% of the patients (one patient used Azopt® and another used Trusopt®). Combination therapies combining timolol maleate with either a carbonic anhydrase inhibitor (Cosopt®) or an alpha-adrenergic inhibitor (Combigan®) were also prescribed for two patients (16.6%).
Figure 1.
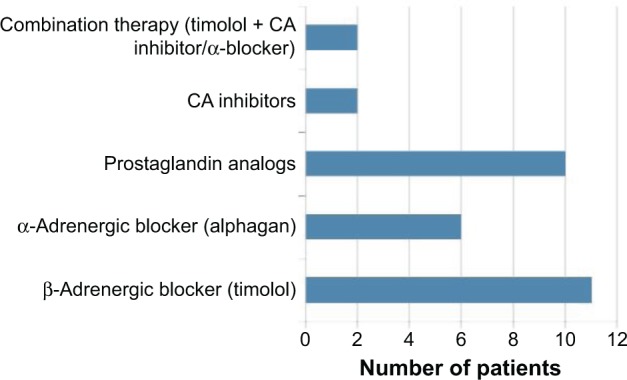
Distribution of IOP-lowering eyedrops used to treat glaucoma.
Notes: Timolol maleate, a beta-adrenergic blocker, was the commonest IOP-lowering topical eyedrop used in these patients, followed by prostaglandin analogs and alpha-adrenergic blockers. In comparison, combination therapy and carbonic anhydrase inhibitors were used much less frequently in these patients.
Abbreviations: IOP, intraocular pressure; CA, carbonic anhydrase.
For the 14 eyes that underwent trabeculectomy surgery with adjunctive MMC, a sponge soaked with MMC was applied at a concentration of 0.4 mg/mL for 3 minutes underneath the conjunctiva overlying the area of the scleral flap. The eyes were then thoroughly irrigated with 50 mL of balanced salt solution.
OSD onset, progress, and treatment
The median interval before onset of dry eye symptoms following trabeculectomy was 13.5 months, with the greatest delay in appearance of OSD being 118.7 months after surgery. Primary symptoms at presentation were, as reported by patients, severe feeling of dryness (66.7%), foreign body or gritty sensation (25.0%), and redness of eye (8.3%) (Table 2). Best corrected visual acuity (BCVA) of 6/12 or better at presentation to the clinic after surgery was noted in half of the eyes treated with MMC during trabeculectomy.
Table 2.
Clinical findings of ocular surface disease
| Clinical findings | |
|---|---|
| Primary symptoms at presentation | |
| Severe feeling of dryness | 66.7% |
| Foreign body/gritty sensation | 25.0% |
| Redness of eye | 8.3% |
| Degree of dry eye disease | |
| TBUT (seconds) | 5.32±3.02 |
| Schirmer I score (mm/5 min) | 6.14±5.16 |
Abbreviation: TBUT, tear breakup time.
On slit-lamp examination, all eyes had punctate epithelial erosions when stained with fluorescein (Figure 2). As defined earlier, TBUT value and Schirmer I score were also used to measure the degree of dry eye disease in these patients at the time of diagnosis. TBUT ranged from 2 seconds to 13 seconds, with a mean of 5.32 seconds (standard deviation [SD] ±3.02 seconds) and mean Schirmer I score was 6.14 mm/5 min (range: 0–17 mm/5 min; SD±5.16 mm/5 min).
Figure 2.
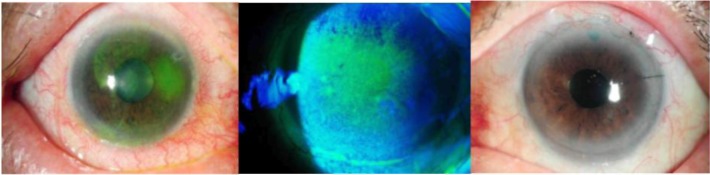
Corneal epitheliopathy in OSD.
Notes: Corneal epitheliopathy in these patients ranged from punctate epithelial erosions to epithelial defects, as seen in three different patients with eyes stained with fluorescein.
Abbreviation: OSD, ocular surface disease.
At the time of presentation to the Dry Eye Clinic, all patients were treated with lubricants, and depending on disease progression and severity of dry eye disease, treatment was stepped up accordingly. Five of the 12 patients (41.6%) required the use of punctal occluders (four temporary and one permanent) to better control their symptoms, and of these, two eyes further required punctal cauterization. Topical steroid eyedrops were also prescribed for three eyes to reduce corneal inflammation and topical cyclosporine eyedrops (Restasis®; Allergan) were prescribed for two eyes. Three patients were noted to have MGD and/or blepharitis, and they were prescribed a short course of doxycycline and were instructed on proper lid hygiene.
After treatment, improvement in BCVA (at least one-line improvement) from presentation to the Dry Eye Clinic until the final visit was observed in seven eyes (46.7%) (Table 3). Median duration of follow-up was 15 months. No improvement in BCVA was noted in three eyes (20%), and worsening of BCVA was noted in two eyes (13.3%). One patient defaulted treatment following his first visit to the Dry Eye Clinic; hence, subsequent BCVA was not available for comparison.
Table 3.
Improvement in VA after treatment (using Snellen chart; N=15)
| VA after treatment | n (%) |
|---|---|
| Improvement in VA | 7 (46.7) |
| Gain ≥4 lines | 2 (28.6) |
| Gain ≥2 lines and <4 lines | 2 (28.6) |
| Gain 1 line | 3 (42.9) |
| No improvement in VA | 3 (20.0) |
| Worsening of VA | 2 (13.3) |
| Lost to follow-up | (6.7) |
Abbreviation: VA, visual acuity.
Discussion
Many patients may require long-term application of lubricating eyedrops, and subsequent escalation of treatment may also be necessary.
To date, there have been no studies reporting the incidence of dry eye disease in patients after trabeculectomy with MMC. Instead, previous studies on outcomes of MMC trabeculectomy have described the occurrence of other complications such as bleb-related endophthalmitis, bleb avascularity, transconjunctival oozing, delayed bleb leaks, and hypotony maculopathy.6–8 MMC is now widely used for surface ablation procedures, such as photorefractive keratectomy or phototherapeutic keratectomy, for refractive and therapeutic treatments, yet questions regarding its long-term safety profile still remain.9 As mentioned in the Introduction, dry eye disease has been reported in patients who have undergone other ocular treatments with MMC. Morales et al10 reported a significantly reduced postoperative endothelial cell count in patients who underwent photorefractive keratectomy with MMC. In another study conducted by Lichtinger et al11 limbal stem cell deficiency (LSCD) was reported in patients after topical MMC was used to treat primary acquired melanosis with atypia. LSCD was reported in patients who were of older age and who received higher concentrations and longer durations of MMC therapy.
Many factors can potentially contribute to the development of OSD in the patients studied. A stable ocular surface is maintained by the presence of a healthy conjunctiva, proper anatomic orientation of the eyelids, and appropriate homeostasis of the corneal epithelial surface and corneoscleral limbus.12 Hence, any disruption to the normal architecture or functions of the conjunctiva, eyelids, and cornea can potentially result in the development of dry eye disease.
The use of MMC may contribute to the ocular surface dysfunction by possibly playing a role in the development of iatrogenic LSCD postsurgery. The antimetabolite activity of MMC targets the actively replicating corneoscleral limbal cells, damaging these cells and thus preventing adequate replacement of the corneal epithelium, which is usually replaced by the stem cells every 3–7 days.12 Chronic use of glaucoma medications has been shown to significantly reduce the percentage of live conjunctival and corneal epithelial cells compared with controls in vitro.13 Coupled with direct microtraumatic insult to the limbus, which occurs during surgery, chronic use of combination medications with MMC applied during surgery can lead to both the breakdown of the corneal epithelium and the development of OSD in susceptible eyes.
Furthermore, surface irregularity of the conjunctiva adjacent to the bleb may also play an important role in the development of OSD in these patients. Dellens have been known to form adjacent to areas of pterygium or filtering blebs.14 These are usually caused by a break in the tear film layer due to local uneven elevations of the cornea, thus disrupting the normal smooth precorneal tear film and wetting of the ocular surface.
Antiglaucoma medications can also pose an additional proinflammatory influence on the conjunctiva, with an increase in subepithelial macrophages, lymphocytes, mast cells, and fibroblasts.15 Preservatives present in these topical antiglaucoma eyedrops, the most common being benzalkonium chloride, have been proven to be toxic to corneal endothelial cells. Studies conducted in Japan incubated human corneal endothelial cells with various types of antiglaucoma medications in the presence and absence of preservatives. Results showed reduced survival of the cells in all medications containing benzalkonium chloride.16,17 Using very-high-frequency ultrasound imaging, corneal epithelium exposed to timolol eyedrops preserved with 0.01% benzalkonium chloride solution also showed significant reduction in epithelial thickness.18
Prior to surgery, the status of the existing ocular surface may also play a part in the development of dry eye disease postoperatively. Preexisting MGD can lead to evaporative dry eye disease if not treated appropriately. Chronic toxicity to conjunctival epithelial cells by topical antiglaucoma medications can also reduce the effectiveness of the conjunctival goblet cells, which function to produce the inner mucin layer of the precorneal tear film.17 Together, these factors can play a major contributory role in compromising the ocular surface preoperatively, which can be exacerbated postoperatively by surgical disruption to the ocular surface and use of the antimetabolite MMC. Preoperative evaluation should include assessment for signs of dry eyes, including abnormal TBUT value and Schirmer I score. A drop in postoperative visual acuity should also alert the attending ophthalmologist to the possible contributing factor of a change in the ocular surface and to determine this with fluorescein staining of the cornea and by performing further tear evaluation.
As our study involved only a retrospective case notes review of patients identified with symptomatic OSD following antimetabolite-augmented trabeculectomy, our sample size may not be entirely representative of all glaucoma patients undergoing trabeculectomy. However, the results in this study highlight and support other reports on the clinical significance of OSD in the glaucoma patient. A prospective study to investigate changes in the ocular surface in glaucoma patients before and after trabeculectomy would further demonstrate the significance of this often-overlooked condition.
Conclusion
MMC has proven utility in reducing the risk of surgical failure following trabeculectomy.19 The use of topical anti-glaucoma medications prior to surgery may also render the ocular surface more susceptible to the intraoperative effects of MMC. In future, it is anticipated that better understanding of the factors affecting the development of OSD in patients after trabeculectomy with MMC can lead to more targeted treatment and appropriate counseling of patients, hence improving the overall safety profile of this drug in glaucoma patients.
Acknowledgments
This study was supported by the National Research Council, Singapore, under grants NMRC/1105/2007, NMRC/1206/2009, and NMRC/CSA/045/2012.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen PP, Yamamoto T, Sawada A, Parrish RK, 2nd, Kitazawa Y. Use of antifibrosis agents and glaucoma drainage devices in the American and Japanese Glaucoma Societies. J Glaucoma. 1997;6(3):192–196. [PubMed] [Google Scholar]
- 3.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 4.Taneri S, Koch JM, Melki SA, Azar DT. Mitomycin-C assisted photorefractive keratectomy in the treatment of buttonholed laser in situ keratomileusis flaps associated with epithelial ingrowth. J Cataract Refract Surg. 2005;31(10):2026–2030. doi: 10.1016/j.jcrs.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Roh DS, Funderburgh JL. Impact on the corneal endothelium of mitomycin C during photorefractive keratectomy. J Refract Surg. 2009;25(10):894–897. doi: 10.3928/1081597X-20090617-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuijts RM, Vernimmen RC, Webers CA. Mitomycin C primary trabeculectomy in primary glaucoma of white patients. J Glaucoma. 1997;6(5):293–297. [PubMed] [Google Scholar]
- 7.Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol. 2006;90(2):175–180. doi: 10.1136/bjo.2005.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields MB, Scroggs MW, Sloop CM, Simmons RB. Clinical and histopathologic observations concerning hypotony after trabeculectomy with adjunctive mitomycin C. Am J Ophthalmol. 1993;116(6):673–683. doi: 10.1016/s0002-9394(14)73465-8. [DOI] [PubMed] [Google Scholar]
- 9.Shah RA, Wilson SE. Use of mitomycin-C for phototherapeutic keratectomy and photorefractive keratectomy surgery. Curr Opin Ophthalmol. 2010;21(4):269–273. doi: 10.1097/ICU.0b013e32833a8c9b. [DOI] [PubMed] [Google Scholar]
- 10.Morales AJ, Zadok D, Mora-Retana R, Martinez-Gama E, Robledo NE, Chayet AS. Intraoperative mitomycin and corneal endothelium after photorefractive keratectomy. Am J Ophthalmol. 2006;142(3):400–404. doi: 10.1016/j.ajo.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Lichtinger A, Pe’er J, Frucht-Pery J, Solomon A. Limbal stem cell deficiency after topical mitomycin C therapy for primary acquired melanosis with atypia. Ophthalmology. 2010;117(3):431–437. doi: 10.1016/j.ophtha.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GS, Holland EJ. Iatrogenic limbal stem cell deficiency: when glaucoma management contributes to corneal disease. J Glaucoma. 2001;10(6):443–445. doi: 10.1097/00061198-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ammar DA, Kahook MY. The effects of combination glaucoma medications on ocular surface epithelial cells. Adv Ther. 2009;26(10):970–975. doi: 10.1007/s12325-009-0076-8. [DOI] [PubMed] [Google Scholar]
- 14.Quaranta L, Pizzolante T. Endophthalmitis after compression sutures for enlarged conjunctival filtration bleb following trabeculectomy. Ophthalmic Surg Lasers Imaging. 2009;40(4):432–433. doi: 10.3928/15428877-20096030-17. [DOI] [PubMed] [Google Scholar]
- 15.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994;112(11):1437–1445. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 16.Ayaki M, Noda Y, Yaguchi S, et al. Cytotoxicity of antiglaucoma ophthalmic solutions for human corneal endothelial cells. Nippon Ganka Gakkai zasshi. 2009;113(5):576–582. [PubMed] [Google Scholar]
- 17.Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008;36(6):553–559. doi: 10.1111/j.1442-9071.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 18.Denoyer A, Ossant F, Arbeille B, et al. Very-high-frequency ultrasound corneal imaging as a new tool for early diagnosis of ocular surface toxicity in rabbits treated with a preserved glaucoma drug. Ophthalmic Res. 2008;40(6):298–308. doi: 10.1159/000134928. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005;(4):CD002897. doi: 10.1002/14651858.CD002897.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


