Abstract
Nitric oxide (NO) and endothelin (ET) produced in endothelial cells are leading molecules which regulate vascular function. Failure of the physiological balance between these two molecules is usually referred to as endothelial dysfunction. ET was initially identified as a potent vasoconstrictive peptide. Three ET isoforms and two ET receptors have been identified. One of the isoforms, ET-1, plays a significant role in many cardiovascular diseases. On the other hand, oxidized low-density lipoprotein (oxLDL) is known to induce endothelial dysfunction. The endothelial receptor for oxLDL was cloned, and named lectin-like oxidized receptor-1 (LOX-1). Activation of LOX-1 generates reactive oxygen species (ROS), and acivates a transcriptional factor, nuclear factor κB (NFκB), resulting in down-regulation of NO and up-regulation of ET-1. LOX-1 might be a key molecule in the generation of endothelial dysfunction. In endothelial dysfunction, ET-1 is an aggravating factor of cardiovascular diseases.
Keywords: Endothelial dysfunction, endothelin, endothelin receptor, endothelin-converting enzyme, oxLDL, LOX-1
The inner wall of blood vessels is covered with a single-cell lining membrane, called endothelium. The endothelium was originally thought to be a simple barrier separating the ciculating blood and the vascular smooth muscle cells. Therefore, discovery of endothelium-derived relaxing factor (EDRF) by Furchgott and Zawadzki in 19801) opened a new avenue in vascular biology. Their results demonstrated the existence of an additional function of the endothelium other than as a simple barrier. Following their discovery, it has become apparent that endothelial cells synthesize and release several important factors which regulate vascular function. It is now thought that the endothelial cell plays an important role in maintaining the homeostatic balance of blood vessels. Any imbalance in the production and release of these substances leads to a variety of vascular diseases, such as hypertension, atherosclerosis and thrombosis.
Discovery of endothelin
The EDRF discovered by Furchgott and Zawadzki was later proven to be nitric oxide. In addition to nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factor were also shown to be EDRFs.
The concept of endothelium-dependent vasodilation was derived in an indirect way based on an isolated vascular-preparation experiment in which the removal of endothelium from various vascular beds rendered them unresponsive to agents such as acetylcholine. In contrast, a factor derived from endothelium which induces vasoconstriction directly has been investigated by several groups.
In 1985, Hickey et al.2) reported that a cultured medium of bovine endothelial cells induced constriction of isolated blood vessels. Subsequently, O’Brien et al.3) demonstrated that the constricting factor is a peptide-like substance.
In 1987, we started purification of this substance. The supernatant from confluent monolayer cultures of porcine aortic endothelial cells caused an endothelium-independent, slow-onset contraction of the vascular bed. Trypsin abolished this activity, suggesting that the constricting factor was a peptide-like substance. We collected the active fraction from the medium of the confluent monolayer cultures of the endothelial cells after anion-exchanger column chromatography and reversed-phased high performance liquid chromatography (HPLC). The amino-acid sequence of this peptide was determined with an automatic peptide sequencer. It consisted of 20 amino-acid residues containing four cysteine residues. The four cysteine residues form two intra-molecular disulfide bonds. The amino-acid sequence of this peptide was further confirmed by the results of a nucleotide sequence of cDNA which was obtained with a synthetic probe of DNA according to the obtained amino-acid sequence. However, the 20-amino acid peptide synthesized according to the results of the sequence analysis initially showed no biological activity. Therefore, we looked again at the nucleotide sequence of the cDNA and realized that the natural peptide is a 21-amino acid peptide, and that the 21st amino acid is tryptophan. Tryptophan is often missed with the sequence analysis. The synthetic 21-amino acid peptide showed complete biological activity, and identical HPLC-profiles to those of the natural peptide. It was a novel vasoconstrictive peptide. Thus we were able to isolate and identify this endothelium-derived contracting factor from the medium of confluent monolayer cultures of porcine aortic endothelial cells, and published the results in 1988.4) The substance was named endothelin (ET).4),5) The discovery of ET stimulated worldwide interest as a result of its potent, long-lasting contraction of isolated vascular beds and its sustained pressor response following a bolus intravenous injection into rats. At the initial stage of the ET research, it was widely believed that ET plays an important role in the maintenance of blood pressure.
Discovery of isoforms of ET
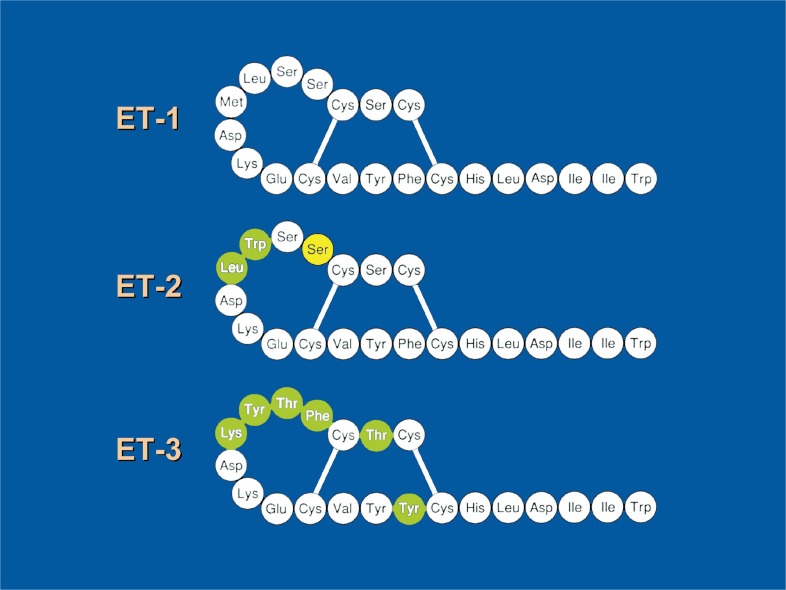
Soon after the discovery of ET, analysis of the gene encoding ET revealed the existence of two other genes encoding an ET-like peptide.6) These three genes are located in different chromosomes. The first discovered ET gene (human) is located on chromosome 6, and the second and third ET genes are located on chromosomes 1 and 20, respectively. The amino acid sequences of these peptides were very similar to that of the first reported ET. Therefore, these three endogenous isoforms were designated as ET-1, ET-2 and ET-3 (Fig. 1). Each of the three peptides consists of the 21 amino-acid residues and is expressed, in different patterns, in various tissues and cells. The predominant ET produced in endothelial cells is ET-1.
Fig. 1.
Isoforms of endogenous endothelin. ET-1 is the ET isolated first from the culture medium of endothelial cells. Leu-6 and Met-7 of ET-1 are replaced by Trp-6(green) and Leu-7(green) in ET-2. Six amino acids (Ser-2, Ser-4, Ser-5, Leu-6, Met-7 and Phe-14) of ET-1 are replaced by six amino acids (Thr-2, Phe-4, Thr-5, Tyr-6, Lys-7, and Tyr-14)(green) in ET-3 respectively. In mouse ET-2, Ser-4(yellow) is replaced by Asn-4.
Interestingly, a group of similar peptides, named sarafotoxins (SRTXs) has been found in the cardiotonic venom of the asp. To date, six 21-amino acid residue SRTXs and a similar snake venom, bibrotoxin, are known to belong to this group.7) These endothelin/sarafotoxin family peptides exhibit similar pharmacological activities. SRTX-b, one of the SRTXs causes positive inotropic action on cardiac muscles, vasoconstriction and atrio-ventricular conduction block. Both mammalian ET and snake venom SRTX evolved from the same ancestral gene but are used differently, for exocrine poison in snakes, and for endocrine signalling in mammals.
Unique biosynthetic pathway of ET
Sequence analysis of porcine ET-1 predicted that mature ET-1 is generated from a precursor via an unusual, previously unknown, proteolytic processing pathway. After the removal of signal peptide, the precursor is selectively processed by an enzyme, furin-like peptidase, to yield a biological inactive intermediate, called big-endothelin(Big-ET). Big-ET is further converted into active ET by a previously unknown endopeptidase, which we called endothelin-converting enzyme (ECE).4),5) Identification of ECE was difficult, because the amount of the enzyme contained in tissues or cells is small. Initially, various peptidases were reported to be candidates for ECE. However, at an early stage, a metallopeptidase inhibitor, phosphoramidon, a potent inhibitor of neprilysin, was shown to inhibit the generation of ET-1 from big-ET-1. Ultimately, this phosphoramidon-sensitive membrane protease was purified from endothelial cells, lung cells and adrenal glands, and the cDNA encoding ECE was cloned respectively by three independent laboratories.8)–10) ECE is structurally similar to neutral endopeptidase. ET-2 and ET-3 are also processed from their own precursors by ECE.
So far, several isoforms of ECE have been reported11),12); four different human isoforms: ECE-1a, ECE-1b, ECE-1c and ECE-1d, which differ in their short N-terminal cytoplasmic domains, were reported. They distribute at different subcellular sites. ECE-1a is abundant in endothelial cells, resides within intracellular secretory vesicles and is constitutively transported to the cell surface. By contrast, ECE-1b is located in the intracellular trans-Golgi networks. ECE-1c and ECE-1d are localized at the cell surface and might act as ectoenzymes. ECE-2 distributes intracellularly and is activated under acidic conditions. The existence of additional ECE isoforms is possible because high levels of ET-1 have been reported in ECE-1 and ECE-2 double knockout mice.13)
Identification of the ET receptor
At the early stage of ET research, dissociation of the potency order of the three ET isopeptides was reported in a variety of systems. In general, responses can be divided into two groups according to the pharmacological potency and radioligand binding of the three isopeptides. Indeed, subsequently, two different but similar cDNAs encoding for these two receptors were cloned by different groups.14),15) Both receptors belong to the family of heptahelical G-protein coupled receptors. These receptors were designated ETA and ETB because of their different affinity for endogeneous ligands. ETA has a higher affinity for ET-1 and ET-2 than for ET-3. In contrast, ETB shows equal affinity to all three endogeneous ligands. These two receptors are distributed in various tissues and organs at different levels, suggesting that endogenous ET is multifunctional.5) It was also demonstrated that a single copy of the human ETA and ETB receptor genes localize to chromosomes 4 and 13, respectively.
Inhibitor of the ET system
Since ET showed a potent vasoconstriction in isolated vascular beds and elicited unique sustained and elevated blood pressure when it was injected intravenously into animals, ET-1 was thought to play an important role in the maintenance of blood pressure. Therefore, many pharmaceutical companies became interested in inhibitors of this ET system. At an early stage, a report on an ETA receptor antagonist cyclic pentapeptide, BQ-123, was published.16) The original compound of BQ-123 was BE1857A which was found in the fermentation broth of the microbe, Streptomyces misakiensis. Extensive structure and activity studies of BE1857A led to the formation of the potent ETA antagonist, BQ-123. Another group reported a potent ETA antagonist, FK139317,17) derived from the same original compound as was BQ-123. The structure-activity relationship analysis of ET led to discovery of potent peptide ETA antagonists, such as TTA386,18) at the first stage of ET research. Also, modification of the C-terminus of ET led to the discovery of a potent linear tripeptide ETB antagonist BQ788.19)
The first non-peptide ET receptor antagonist to be effective following oral administration was found in sulfonamide derivatives. A structure-activity relationship study with this compound provided a non-selective ET receptor antagonist, bosentan.20) Another series of ET antagonists were found succesively in natural products and chemical compound libraries.
The peptide ETA antagonist, BQ123, and the ETB antagonist, BQ788, were widely used for experiments, thus contributing greatly to ET research. The non-selective ET antagonist, bosentan, has been widely used in basic and clinical research, and has provided important information. Bosentan has been approved by the Food and Drug Administration (FDA) of the USA for clinical use in the treatment of pulmonary hypertension.
Physiological significance of ET
Because of its unique pharmacological properties, most investigators have thought that the ET-system plays an important role in the maintenance of blood pressure, as does the renin-angiotensin system. However, numerous studies subsequently published have demonstrated that the plasma ET-1 level is very low, suggesting that ET-1 is not a circulating hormone, but rather a local hormone. ET-1 is produced in endothelial cells, secreted preferentially to the basal side of the endothelium and acts on underlying smooth muscles via ETA, inducing vasoconstriction. Also the released ET-1 acts on the neighboring endothelial cells in an autocrine and paracrine manner via ETB, releasing relaxing factors, such as prostacycline and nitric oxide.5) These relaxing factors in turn act on the underlying smooth muscles, inducing relaxation. Thus ET-1 released from the endothelium induces both constriction and relaxation. The data accumulated so far has demonstrated that endogenous ET-1 is involved in the regulation of peripheral blood flow, mostly via ETA on smooth muscle.5),21),22) In contrast, analysis of the blood pressure of mice with the knockout gene encoding the ET-1 or ETB receptor suggested that ET-1 also induces a reduction in blood pressure through ETB on endothelial cells.5),23),24)
To complicate matters further, it was also reported that activated ETB on nephrons suppresses sodium intake through the epithelial sodium channels of the inner medullary collecting duct of the nephrons.25) Therefore, blood pressure increases in ETB-defficient mice may be partly due to the accumulation of sodium ions.26)
Following the discovery of ET, many investigators measured the plasma levels of ET-1 and found elevated levels, not only in cardiovascular diseases but also in non-cardiovascular diseases.5) As mentioned above, ET isoforms and ET receptors are distributed in various tissues and organs. These facts have already suggested the multifunctional activity of ET systems in various tissues and cells. Analysis of knockout mice of the genes encoding ET isoforms and ET receptors also provided several unexpected results. Nowadays, it is recognized that the ET-1-ETA system is important in the development of the cranial and cardiac neural crest, and the ET-3-ETB system in the development of melanocytes and enteric neurons derived from the trunk and vagal crest.27)–29) Hirschsprung disease, which is chacterized by megacolon, and Shah-Waardenburg syndrome, which is characterized by a pigmentary disorder and hearing loss, are ascribed to mutations of the ET-3 gene or ETB receptor gene.27)–30) Physiologically, the ET system is involved in both cell growth and cell differentiation. Pathologically, ET is involved in inflammation, cancer growth and metastasis, and morphological abnormalities.27)
Pathophysiological significance of ET in the cardiovascular system
It is now recognized that ET plays crucial roles in several cardiovascular diseases, including chronic heart disease, ischemic heart disease, hypertension, atherosclerosis, pulmonary hypertension, chronic heart failure and cerebrovascular spasm after subarachnoid hemorrhage.5) In these diseases, the circulating level of ET-1 is high in many cases, and investigators have sought diseases for which administration of ET inhibitors would be therapeutically beneficial. Among these diseases, chronic heart failure, essential hypertension and pulmonary hypertension are being investigated extensively.
Endothelial dysfunction and vascular diseases
Thus, ET is now recognized as being involved in the pathological process of vascular diseases. The production of ET and nitric oxide is regulated by the condition of the endothelium. In endothelial dysfunction, the production of nitric oxide is downregulated. In contrast, the production of ET is upregulated. This yin and yang relationship of ET and nitric oxide production maintains the homeostasis of the vascular beds under physiological conditions. Any imbalance of this homeostasis promotes the pathological process of the vascular diseases at their primary stage, leading to a decrease in antithrombotic activity, and an increase in vascular tone and permeability and in cell growth activity.31)
What kind of factor induces endothelial dysfunction? Numerous reports have suggested the generation of reactive oxygen species (ROS) and an increased level of oxidative low-density lipoprotein (oxLDL) as important pathogenic factors. It is generally accepted that the oxidatively modified form of LDL is implicated in the pathogenesis of atherosclerosis32); OxLDL is taken up into the macrophages and smooth muscle cells, and changes these cells into foam cells, leading to the formation of atheroma. Similarly, oxLDL was also demonstrated to be taken up by endothelial cells.33) However, the endothelial receptive site for oxLDL was unknown at the time of their studies. The known oxLDL receptors detected in macrophages or smooth muscle cells cannot be detected in endothelial cells.
In 1990, Kugiyama et al.34) demonstrated that oxLDL impaired the endothelium-dependent relaxation of isolated vascular beds induced by acetylcholine. Non-oxidized LDL does not induce impairment in the presence of an antioxidative reagent, although a previous report showed slight impairment of native LDL in the absence of an antioxidant. These results showed that native LDL is oxidized during an interaction with oxygen, and ROS possibly activates endothelial cells via endothelial receptive sites for oxLDL, leading to endothelial dysfunction, i.e., down-regulation of nitric oxide production and, presumably, up-regulation of ET production. These results suggest that the endothelial cell has a specific receptor for oxLDL, which does not react to native LDL.
Discovery of an endothelial receptor for oxLDL and its expression
Following these results, cDNA encoding a novel endothelial receptor for oxLDL was cloned by Sawamura et al. in 1997.35) The receptor is a type-II membrane protein belonging to a family of C-type lectin-like molecules, and was named lectin-like oxidized LDL receptor (LOX-1).
LOX-1 is expressed at a low level in a statical condition of endothelial cells, but is induced by proinflammatory cytokines and vasoconstrictive peptides in vitro and in proatherogenic conditions in vivo, such as hypertension, diabetes and hyperlipidemia.31) Various substances that induce oxdative stress, such as angiotensin II and homocysteine were shown to induce LOX-1 expression. On the other hand, LDL is oxidized by oxidative stress, leading to the generation of oxLDL, which further up-regulates LOX-1 in the endothelial cells via activation of LOX-1, although native LDL does not do this. These results suggest that the binding of oxLDL to LOX-1 enhances the uptake of oxLDL even more, resulting in the potential for further endothelial dysfunction.
LOX-1 regulates expression of NO and ET
In 2000, Cominacini et al. demonstrated that the binding of oxLDL to the LOX-1 induced production of the super-oxide anion and activated NFκB, a redox-sensitive transcription factor.36) They demonstrated that oxLDL reduced NO production from the endothelial cells via generation of reactive oxygen. Vitamin C reversed the reduction of NO production induced by oxLDL, suggesting that oxidative stress reduces NO production. In contrast to the reduction in NO production by oxLDL, ET-1 production from endothelial cells is enhanced by oxLDL,31),37) probably via activation of NFκB. (Fig. 2) Antibody against LOX-1, which blocks LOX-1 activity, reduced the increase in production of ET-1 by oxLDL, suggesting that the production of ET-1 is mediated by LOX-1. Superoxide dismutase (SOD) also reduces this enhanced production of ET-1 by oxLDL, while it does not affect the enhanced expression of ET-1 induced by the inflammation-related membrane protein, CD40L. These results suggest the involvement of ROS as well in the elevation of ET-1 by oxLDL.
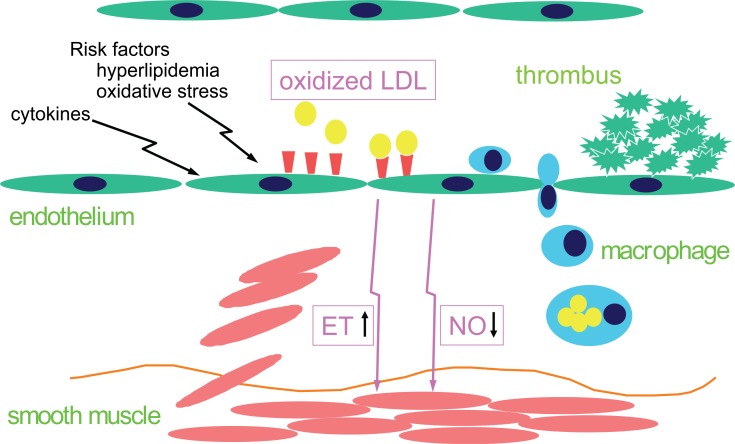
Fig. 2.
Disruption of vascular homeostasis via endothelial LOX-1. OxLDL, inflammatory cytokines and risk factors for cardiovascular diseases induce the expression of LOX-1(shown as brown wedges) on endothelial cells, resulting in down-regulation of NO via the generation of ROS and up-regulation of ET-1 via activation of NFκB (endothelial dysfunction). Activation of LOX-1 also induces expression of chemokines and adhesive molecules, resulting in macrophage infiltration into the vascular wall and thrombus formation.
LOX-1 expressed in the endothelial cell was shown to bind also to platelets, leading to a phenotype change of the endothelium as with oxLDL.31),38) The endothelial cells bound by activated platelets enhanced the production of superoxide anions and ET-1. This endothelial activation was also blocked by anti-LOX-1 antibody.
It is now understood that in endothelial cells the activation of redox-sensitive transcription factors, including NFκB, by oxidative stress promotes upregulation of ET-1 as well as upregulation of the adhesive molecules and chemokines.
Importantly, LOX-1 is now known as a novel adhesive molecule involved in leukocyte recruitment and inflammation that further increases oxidative stress. In a model of low-dose endotoxin-induced uveitis, leucocytes interacted with and bind to the inner wall of the retinal vein.31),39) Anti-LOX-1 antibody suppressed the binding of leucocytes as well as leukocyte infiltration and protein exudation, suggesting the LOX-1 functions as a vascular endothelial ligand for leukocytes. Actually, recombinant LOX-1 protein binds leukocytes in vitro in a flow condition.
In summary, these results support the concept that oxLDL induces NO reduction via oxidation of NO via the generation of reactive oxygen species, and enhances ET-1 production as a result of phenotypic change in the endothelium through activation of NFκB. These properties of LOX-1 can be understood to change endothelial function, which increases prothrombotic and proinflammatory activity.
Conclusion
ET was initially identified as a novel endothelial vasoconstrictor. Three ET isoforms were identified, namely ET-1, ET-2 and ET-3. The vascular endothelial cell exclusively produces ET-1. Two distinct ET receptors, ETA and ETB, were cloned. In the cardiovascular system, the ET-1-ETA system is implicated mostly in vascular homeostasis. Recently, ET-1 was recognized as an aggravating factor in several cardiovascular diseases, interacting with LOX-1, a novel endothelial receptor for oxLDL. LDL is oxidized under oxidative stress. OxLDL binds to LOX-1, generating superoxide anions and activating NFκB in the endothelial cells. The increase in superoxide anions accelerates inactivation of NO. On the other hand, activated NFκB induces upregulation in the expression of vasoconstrictive molecules, including ET, adhesion molecules and chemokines, resulting in an increase in vascular tone, thus promoting the infiltration of monocytes into the vessel wall and the release of additional proinflammatory signals. The reduction in NO and production of ET by oxLDL in the endothelial cell generates and promotes vascular diseases, such as atherosclerosis. In this process, LOX-1 plays a pivotal role in changing endothelial functions, such as prothrombotic and proinflamatory activity.
So far, accumulating evidence has shown the involvement of LOX-1 in vascular diseases, including atherosclerosis, arteriosclerosis after cardiac transplantation, restenosis after percutaneous transluminal coronary angioplasty (PTCA), myocardial infarction, angina pectoris, thrombosis and inflammation, including arthritis and uveitis. Interestingly, the plasma ET-1 level increases in these diseases.
Oxidative stress induces oxLDL, which leads to endothelial dysfunction, including the upregulation of LOX-1. Activation of LOX-1 induces the production of ET-1 and other inflammatory factors. These factors aggravate vascular diseases.
Abbreviation.
- NO
nitric oxide
- ET
endothelin
- oxLDL
oxidized low-density lipoprotein
- LOX-1
lectin-like oxLDL receptor-1
- ROS
reactive oxygen species
- NFκB
nuclear factor kappa B
- EDRF
endothelium-derived relaxing factor
- HPLC
high performance liquid chromatography
- ECE
endothelin-converting enzyme.
- SRTX
sarafotoxin
- SOD
super oxide dismutase
- PTCA
percutaneous transluminal coronary angioplasty.
Profile
Tomoh Masaki was born in 1934. In 1963, after graduation from the Faculty of Medicine, University of Tokyo, and finishing internship training, he started his career with studies on structural proteins of muscle at Professor Setsuro Ebashi’s laboratory. He established α-actinin as a new protein of muscle and also found M-protein, a new protein of muscle, during the late 1960s and the early 1970s. He also demonstrated the existence of several types of myosin, troponin and/or alpha-actinin in different types of muscles, and that these types of muscle protein change during differentiation of muscle. These results were highly motivating in the study of the molecular biology of muscle. The whole primary sequence of several muscle proteins, including smooth muscle myosin heavy chain, was determined at his laboratory. He was promoted to Professor of Pharmacology, Institute of Basic Medical Sciences, University of Tsukuba in 1975, and moved, as Professor of Pharmacology, to the Faculty of Medicine, Kyoto University in 1991. During this period, he focused mostly on the investigation of smooth muscle. In 1987∼1990, a potent endothelial vasoconstrictive peptide, endothelin, and its receptor was discovered at his laboratory in Tsukuba. In 1997, an endothelial receptor for oxLDL was found in his laboratory at Kyoto. These vasoactive factors are thought to play important roles in physiology and pathophysiology of vascular beds, Therefore many investigators in the world are now interested in this problem. In 1997, he became the Director of the Research Institute of the National Cardiovascular Center. In 2003, he assumed the position of President, at Osaka Seikei University.

References
- 1.Furchgott, R. F., and Zawadzki, J. V. (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376. [DOI] [PubMed] [Google Scholar]
- 2.Hickey, K. A., Rubanyi, G. M., Paul, R. J., and Highsmith, R. F. (1985) Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am. J. Physiol. 248, C550–C556. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien, R. F., Robbins, R. J., and McMurtry, I. F. (1987) Endothelial cells in culture produce a vasoconstrictor substance. J. Cell. Physiol. 132, 263–270. [DOI] [PubMed] [Google Scholar]
- 4.Yanagisawa, M, Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K., and Masaki, T. (1988) A novel potent vasoconstrictor peptide produced by vascularendothelial cells. Nature 332, 411–415. [DOI] [PubMed] [Google Scholar]
- 5.Masaki, T. (2004) Historical review: Endothelin. Trends Pharmacol. Sci. 25, 219–224. [DOI] [PubMed] [Google Scholar]
- 6.Inoue, A., Yanagisawa, M., Kimura, S., Kasuya, Y., Miyauchi, T., Goto, K., and Masaki, T. (1989) The human endothelin family: Three structurally and pharmacologically distinct isopeptides by three separate genes. Proc. Natl. Acad. Sci. USA 86, 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochva, E. (2002) Atractaspis (Serpentes, Atractaspididae) the burrowing asp; a multidisciplinary minireview. Bull. Nat. Hist. Mus. Lond. (Zool.) 68, 91–92. [Google Scholar]
- 8.Shimada, K., Takahashi, M., and Tanzawa, K. (1994) Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J. Biol. Chem. 269, 18275–17278. [PubMed] [Google Scholar]
- 9.Xu, D., Emoto, N., Giaid, A., Slaughter, C., Kaw, S., deWit, D., and Yanagisawa, M. (1994) ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78, 473–485. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt, M., Kröger, B., Jacob, E., Seulberger, H., Subkowski, T., Otter, R., Meyer, T., Schmalzing, G., and Hillen, H. (1994) Molecular characterization of human and bovine endothelin-converting enzyme (ECE-1). FEBS Lett. 356, 238–243. [DOI] [PubMed] [Google Scholar]
- 11.Coder, R. (2001) Identity of endothelin-converting enzyme and other targets for the therapeutic regulation of endothelin biosynthesis. InEndothelin and Its Inhibitors (ed. Warner T. D.). Springer, Berlin, Heidelberg, New York, pp. 35–67. [Google Scholar]
- 12.Basttini, B., and Jeng, A. Y. (2001) Endothelin-converting enzyme inhibitors and their effects. InEndothelin and Its Inhibitors (ed. Warner T. D.). Springer, Berlin, Heidelberg, New York, pp. 155–208. [Google Scholar]
- 13.Yanagisawa, H., Hammer, R. E., Richardson, J. A., Emoto, N., Williams, S. C., Takeda, S., Clouthier, D. E., and Yanagisawa, M. (2000) Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J. Clin. Invest. 105, 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai, H., Hori, S., Aramori, I., Ohkubo, H., and Nakanishi, S. (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348, 730–732. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai, T., Yanagisawa, M., Takuwa, Y., Miyazaki, H., Kimura, S., Goto, K., and Masaki, T. (1990) Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endotheline receptor. Nature 348, 732–735. [DOI] [PubMed] [Google Scholar]
- 16.Ihara, M., Noguchi, K., Saeki, T., Fukuroda, T., Tsuchida, S., Kimura, S., Fukami, T., Ishikawa, K., Nishikibe, M., and Yano, M. (1992) Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 50, 247–255. [DOI] [PubMed] [Google Scholar]
- 17.Aramori, I., Nirei, H., Shoubo, M., Sogabe, K., Nakamura, K., Kojo, H., Notsu, Y., Ono, T., and Nakanishi, S. (1993) Subtype selectivity of a novel endothelin antagonist, FR139317, for the two endothelin receptors in transfected Chinese hamster ovary cells. Mol. Pharmacol. 43, 127–131. [PubMed] [Google Scholar]
- 18.Kitada, C., Ohtaki, T., Masuda, Y., Masuo, Y., Nomura, H., Asami, T., Matsumoto, Y., Satou, M., and Fujino, M. (1993) Design and synthesis of ETA receptor antagonists and study of ETA receptor distribution. J. Cardiovasc. Pharmacol. 22 (Suppl. 8), S128–S131. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa, K., Ihara, M., Noguchi, K., Mase, T., Mino, N., Saeki, T., Fukuroda, T., Fukami, T., Ozaki, S., Nagase, T.et al. (1994) Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. USA 91, 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clozel, M., Breu, V., Gray, G. A., Kalina, B., Löffler, B.-M., Burri, K., Cassal, J.-M., Hirth, G., Muller, M., Neidhart, W., and Ramuz, H. (1994) Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 270, 228–235. [PubMed] [Google Scholar]
- 21.Haynes, W. G., Ferro, C. J., O’Kane, K. P. J., Somerville, D., Lomax, C. C., and Webb, D. J. (1996) Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93, 1860–1870. [DOI] [PubMed] [Google Scholar]
- 22.Verhaar, M. C., Strachan, F. E., Newby, D. E., Cruden, N. L., Koomans, H. A., Rabelink, T. J., and Webb, D. J. (1998) Endothelin-A receptor antagonist mediated vasodilataion is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97, 752–756. [DOI] [PubMed] [Google Scholar]
- 23.Kurihara, Y., Kurihara, H., Suzuki, H., Kodama, T., Maemura, K., Nagai, R., Oda, H., Kuwaki, T., Cao, W., Kamada, N.et al. (1994) Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368, 703–710. [DOI] [PubMed] [Google Scholar]
- 24.Kuwaki, T., Kurihara, H., Cao, W. H., Kurihara, Y., Unekawa, M., Yazaki, Y., and Kumada, M. (1997) Physiological role of brain endothelin in the central autonomic control from neuron to knockout mouse. Prog. Neurobiol. 51, 545–579. [DOI] [PubMed] [Google Scholar]
- 25.Pollock, D. M. (2001) Endothelin and the kidney. InEndothelin and Its Inhibitors (ed. Warner T. D.). Springer, Berlin, Heidelberg, New York, pp. 477–501. [Google Scholar]
- 26.Ohuchi, T., Kuwaki, T., Ling, G.-Y., Dewit, D., Ju, K.-H., Onodera, M., Cao, W.-H., Yanagisawa, M., and Kumada, M. (1999) Elevation of blood pressure by genetic and pharmacological disruption of the ETB receptor in mice. Am. J. Physiol. 276, R1040–R1077. [DOI] [PubMed] [Google Scholar]
- 27.Kurihara, H., Kurihara, Y., and Yazaki, Y. (2001) Lessons from gene deletion of endothelin systems. InEndothelin and Its Inhibitors (ed. Warner T. D.). Springer, Berlin, Heidelberg, New York, pp. 141–154. [Google Scholar]
- 28.Clouthier, D. E., Hosoda, K., Richardson, J. A., Williams, S. C., Yanagisawa, H., Kuwaki, T., Kumada, M., Hammer, R. E., and Yanagisawa, M. (1998) Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125, 813–824. [DOI] [PubMed] [Google Scholar]
- 29.Baynash, A. G., Hosoda, K., Giaid, A., Richardson, J. A., Emoto, N., Hammer, R, E., and Yanagisawa, M. (1994) Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79, 1277–1285. [DOI] [PubMed] [Google Scholar]
- 30.Puffenberger, E. G., Hosoda, K., Washington, S. S., Nakao, K., deWit, D., Yanagisawa, M., and Chakravarti, A. (1994) A mis-sense mutation of the endothelin-B receptor gene in multi-genic Hirschsprung’s disease. Cell 79, 1257–1266. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai, K., and Sawamura, T. (2003) Stress and vascular responses: Endothelial dysfunction via lectin-like oxidized low-density lipoprotein receptor-1: Close relationships with oxidative stress. J. Pharmacol. Sci. 91, 182–186. [DOI] [PubMed] [Google Scholar]
- 32.Ross, R. (1986) The pathogenesis of atherosclerosis-an update. N. Engl. J. Med. 314, 488–500. [DOI] [PubMed] [Google Scholar]
- 33.Kume, N., Arai, H., Kawai, C., and Kita, T. (1991) Receptors for modified low-density lipoproteins on human endothelial cells: different recognition for acetylated low-density lipoprotein and oxidized low-density protein. Biochim. Biophys. Acta 1091, 63–67. [DOI] [PubMed] [Google Scholar]
- 34.Kugiyama, K., Kerns, S. A., Morrisett, J. D., Roberts, R., and Henry, P. D. (1990) Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 344, 160–162. [DOI] [PubMed] [Google Scholar]
- 35.Sawamura, T., Kume, N., Aoyama, T., Moriwaki, H., Hoshikawa, H., Aiba, Y., Tanaka, T., Miwa, S., Katsura, Y., Kita, T., and Masaki, T. (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73–77. [DOI] [PubMed] [Google Scholar]
- 36.Cominacini, L., Fratta Pasini, A., Garbin, U., Davoli, A., Tosetti, M. L., Campagnola, M., Rigoni, A., Pastorino, A. M., Lo Cascio, V., and Sawamura, T. (2000) Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-κB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 275, 12633–12638. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai, K., Cominacini, L., Garbin, U., Fratta Pasini, A, Sasaki, N., Takuwa, Y., Masaki, T., and Sawamura, T. (2004) Induction of endothelin-1 production in endothelial cells via co-operative action between CD40 and lectin-like oxidized LDL receptor (LOX-1). J. Cardiovasc. Pharmacol. 44, S173–S180. [DOI] [PubMed] [Google Scholar]
- 38.Kakutani, M., Masaki, T., and Sawamura, T. (2000) A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc. Natl. Acad. Sci. USA 97, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honjo, M., Nakamura, K., Yamashiro, K., Kiryu, J., Tanihara, H., McEvoy, L. M., Honda, Y., Butcher, E. C., Masaki, T., and Sawamura, T. (2003) Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc. Natl. Acad. Sci. USA 100, 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]