Abstract
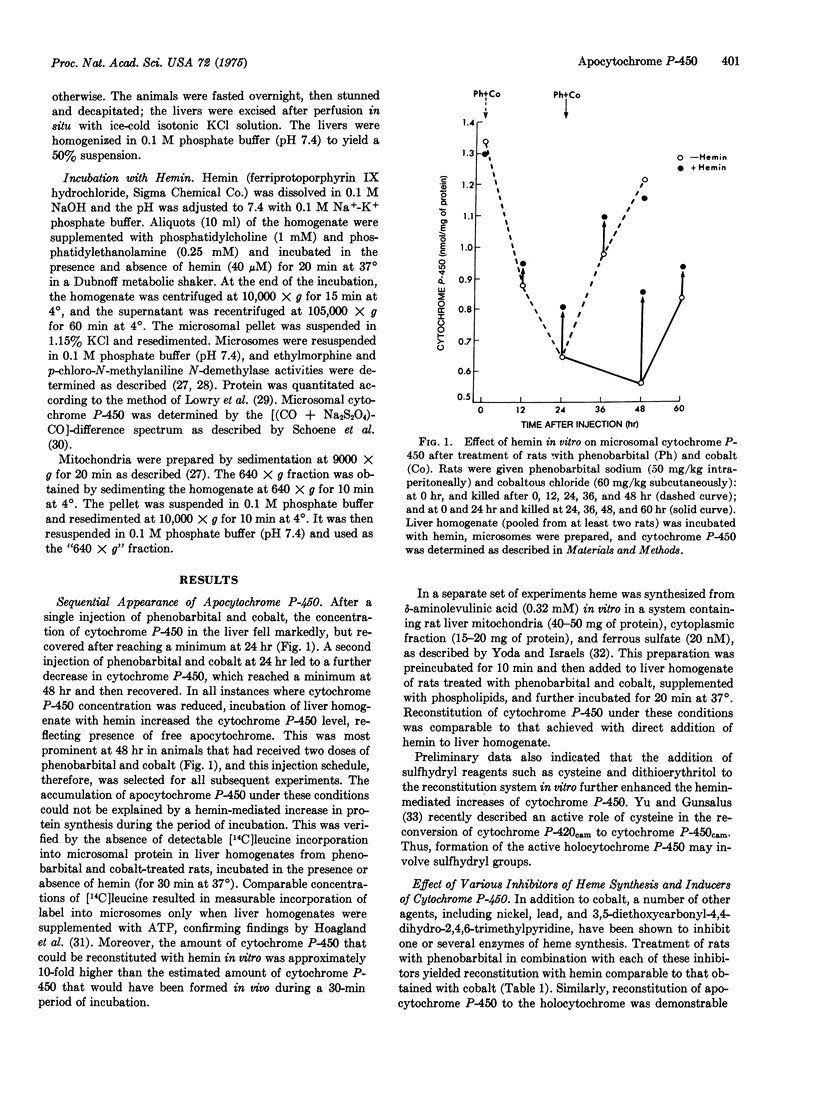
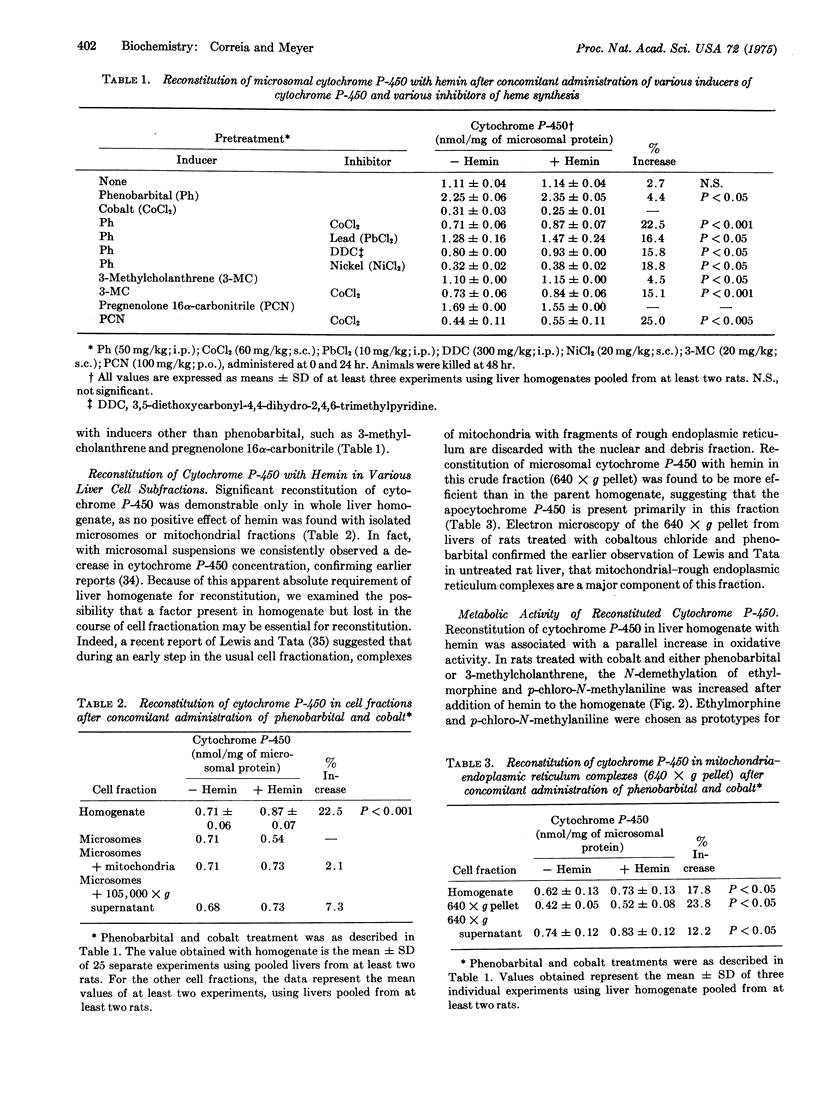
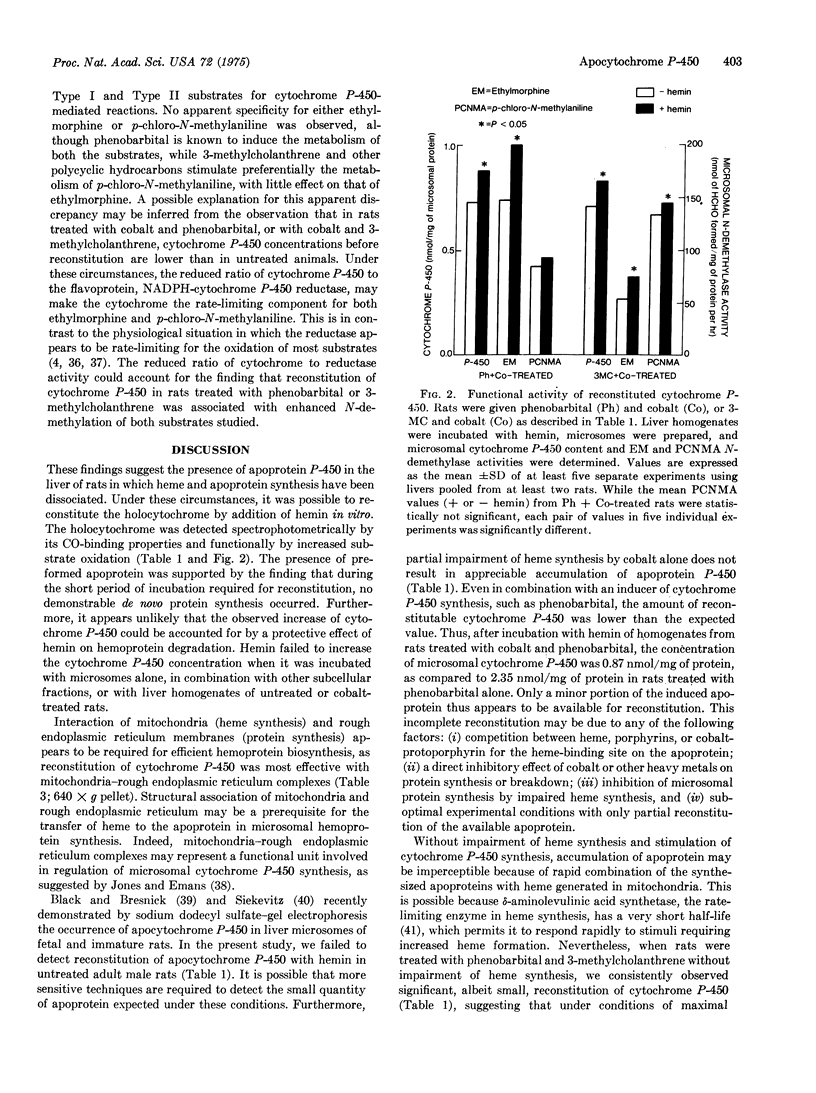
Synthesis of microsomal cytochrome P-450 in rat liver requires synthesis of apoprotein in rough endoplasmic reticulum and of heme in mitochondria. Dissociation of apoprotein and heme synthesis by concomitant treatment of rats with inducers of cytochrome P-450 (i.e., phenobarbital) and inhibitors of heme synthesis (i.e., cobalt) resulted in a relative excess of apocytochrome P-450. Under these circumstances, it was possible to reconstitute the holocytochrome by addition of hemin in vitro. The holocytochrome was detected spectrophotometrically by its CO-binding properties and functionally by its increased oxidative activity. Heme-mediated reconstitution was most efficient in cell fractions rich in mitochondria-rough endoplasmic reticulum complexes (640 times g fraction), suggesting that the structural association of these two organelles may represent a functional unit essential for the synthesis of holocytochrome P-450. These findings indicate that phenobarbital-mediated induction of apocytochrome P-450 is independent of heme synthesis. It is suggested that synthesis of the apocytochrome may be the primary and rate-limiting event in the formation of cytochrome P-450.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black O., Jr, Bresnick E. Ontogenetic changes of proteins of endoplasmic reticulum. J Cell Biol. 1972 Mar;52(3):733–742. doi: 10.1083/jcb.52.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black O., Jr, Cantrell E. T., Buccino R. J., Bresnick E. Effects of 3-methylcholanthrene administration on the proteins of endoplasmic reticulum. Biochem Pharmacol. 1971 Nov;20(11):2989–2998. doi: 10.1016/0006-2952(71)90103-1. [DOI] [PubMed] [Google Scholar]
- CONNEY A. H., GILMAN A. G. PUROMYCIN INHIBITION OF ENZYME INDUCTION BY 3-METHYLCHOLANTHRENE AND PHENOBARBITAL. J Biol Chem. 1963 Nov;238:3682–3685. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Correia M. A., Mannering G. J. Reduced diphosphopyridine nucleotide synergism of the reduced triphosphopyridine nucleotide-dependent mixed-function oxidase system of hepatic microsomes. I. Effects of activation and inhibition of the fatty acyl coenzyme A desaturation system. Mol Pharmacol. 1973 Jul;9(4):455–469. [PubMed] [Google Scholar]
- Correia M. A., Mannering G. J. Reduced diphosphopyridine nucleotide synergism of the reduced triphosphopyridine nucleotide-dependent mixed-function oxidase system of hepatic microsomes. II. Role of the type I drug-binding site of cytochrome P-450. Mol Pharmacol. 1973 Jul;9(4):470–485. [PubMed] [Google Scholar]
- Druyan R., Jakovcic S., Rabinowitz M. Studies of cytochrome synthesis in rat liver. Biochem J. 1973 Jun;134(2):377–385. doi: 10.1042/bj1340377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druyan R., Kelly A. The effect of exogenous -aminolaevulinate on rat liver haem and cytochromes. Biochem J. 1972 Oct;129(5):1095–1099. doi: 10.1042/bj1291095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R. W., Shigematsu A., Schenkman J. B. The contribution of the microsomal electron transport pathway to the oxidative metabolism of liver. Adv Enzyme Regul. 1970;8:121–130. doi: 10.1016/0065-2571(70)90012-9. [DOI] [PubMed] [Google Scholar]
- GELBOIN H. V., BLACKBURN N. R. THE STIMULATORY EFFECT OF 3-METHYLCHOLANTHRENE ON MICROSOMAL AMINO ACID INCORPORATION AND BENZPYRENE HYDROXYLASE ACTIVITY AND ITS INHIBITION BY ACTINOMYCIN D. Biochim Biophys Acta. 1963 Aug 20;72:657–660. [PubMed] [Google Scholar]
- Gigon P. L., Gram T. E., Gillette J. R. Effect of drug substrates on the reduction of hepatic microsomal cytochrome P-450 by NADPH. Biochem Biophys Res Commun. 1968 May 23;31(4):558–562. doi: 10.1016/0006-291x(68)90514-7. [DOI] [PubMed] [Google Scholar]
- Gigon P. L., Gram T. E., Gillette J. R. Studies on the rate of reduction of hepatic microsomal cytochrome P-450 by reduced nicotinamide adenine dinucleotide phosphate: effect of drug substrates. Mol Pharmacol. 1969 Mar;5(2):109–122. [PubMed] [Google Scholar]
- Gillette J. R., Davis D. C., Sasame H. A. Cytochrome P-450 and its role in drug metabolism. Annu Rev Pharmacol. 1972;12:57–84. doi: 10.1146/annurev.pa.12.040172.000421. [DOI] [PubMed] [Google Scholar]
- González-Cadavid N. F., Wecksler M., Bravo M. Stimulating effect of haemin on the synthesis of cytochrome c by liver slices. FEBS Lett. 1970 Apr 16;7(3):248–250. doi: 10.1016/0014-5793(70)80172-7. [DOI] [PubMed] [Google Scholar]
- Grayzel A. I., Fuhr J. E., London I. M. Effects of inhibitors of protein synthesis on the synthesis of heme in rabbit reticulocytes. Biochem Biophys Res Commun. 1967 Sep 7;28(5):705–710. doi: 10.1016/0006-291x(67)90373-7. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOAGLAND M. B., STEPHENSON M. L., SCOTT J. F., HECHT L. I., ZAMECNIK P. C. A soluble ribonucleic acid intermediate in protein synthesis. J Biol Chem. 1958 Mar;231(1):241–257. [PubMed] [Google Scholar]
- Hara T., Minakami S. Presence of apo-cytochrome beta 5 in microsomes. Incorporation of radioactive heme to the cytochrome in vitro. J Biochem. 1970 May;67(5):741–743. doi: 10.1093/oxfordjournals.jbchem.a129303. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Scharf M. B., Vessel E. S. Role of RNA in induction of hepatic microsomal mixed function oxidases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):704–707. doi: 10.1073/pnas.71.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Coordinate synthesis of heme and apoenzyme in the formation of tryptophan pyrrolase. Science. 1966 Oct 28;154(3748):501–503. doi: 10.1126/science.154.3748.501. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Marver H. S. Porphyrin biosynthesis. Enhancement of the fractional catabolic rate of microsomal haem in chemically induced porphyria. S Afr Med J. 1971 Sep 25;:175–177. [PubMed] [Google Scholar]
- Negishi M., Omura T. Presence of apo-cytochrome beta 5 in microsomes from rat liver. J Biochem. 1970 May;67(5):745–747. doi: 10.1093/oxfordjournals.jbchem.a129304. [DOI] [PubMed] [Google Scholar]
- Omura T., Siekevitz P., Palade G. E. Turnover of constituents of the endoplasmic reticulum membranes of rat hepatocytes. J Biol Chem. 1967 May 25;242(10):2389–2396. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene B., Fleischmann R. A., Remmer H., von Oldershausen H. F. Determination of drug metabolizing enzymes in needle biopsies of human liver. Eur J Clin Pharmacol. 1972 Mar;4(2):65–73. doi: 10.1007/BF00562499. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. The differentiation of rat liver endoplasmic reticulum membranes: apo--cytochrome P450 as a membrane protein. J Supramol Struct. 1973;1(6):471–489. doi: 10.1002/jss.400010604. [DOI] [PubMed] [Google Scholar]
- Yoda B., Israels L. G. Transfer of heme from mitochondria in rat liver cells. Can J Biochem. 1972 Jun;50(6):633–637. doi: 10.1139/o72-087. [DOI] [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Cytochrome P-450cam. II. Interconversion with P-420. J Biol Chem. 1974 Jan 10;249(1):102–106. [PubMed] [Google Scholar]


