Abstract
Angiogenesis and osteogenesis are critically linked, though the role of angiogenesis is not well understood in osteogenic mechanical loading. In this study, either damaging or non-damaging cyclic axial compression was used to generate woven bone formation (WBF) or lamellar bone formation (LBF), respectively, at the mid-diaphysis of the adult rat forelimb. αvβ3 integrin targeted nanoparticles or vehicle was injected intravenously following mechanical loading. β3 integrin subunit expression on vasculature was maximal 7 days after damaging mechanical loading, but was still robustly expressed 14 days after loading. Accordingly, targeted nanoparticle delivery in WBF loaded limbs was increased compared to non-loaded limbs. Vascularity was dramatically increased after WBF loading (+700% on day 14) and modestly increased after LBF loading (+50% on day 14). This increase in vascularity was inhibited by nanoparticle treatment in both WBF and LBF loaded limbs at days 7 and 14 after loading. Decreased vascularity led to diminished woven, but not lamellar, bone formation. Decreased woven bone formation resulted in impaired structural properties of the skeletal repair, particularly in post-yield behavior. These results demonstrate that αvβ3 integrin mediated angiogenesis is critical for recovering fracture resistance following bone injury, but is not required for bone modeling after modest mechanical strain.
Keywords: mechanical loading, bone formation, angiogenesis, nanoparticles, stress fracture
1. INTRODUCTION
Angiogenesis, the process by which new vessels extend from the existing vascular system, is crucial for osteogenesis, the production of new bone tissue. In fact, many osteogenic processes fail without sufficient angiogenesis, including stress fracture repair(1), distraction osteogenesis(2–4), fracture healing(5,6), and skeletal development(7,8). Repetitive mechanical loading of the skeleton is a potent osteogenic stimulus, having the ability to induce either woven or lamellar bone formation. However, the role of angiogenesis and the mechanisms that govern the process in this context are not well understood.
Cyclic mechanical loading at hyperphysiological strain levels leads to the accumulation of skeletal fatigue damage, stimulating the formation of non-endochondral woven bone during the repair process(9). In the rat forelimb, a single bout of damaging, cyclic loading has been shown to reproducibly generate a non-displaced stress fracture that leads to periosteal woven bone formation at the ulnar mid-diaphysis(10). In this scenario, woven bone formation is associated with the strong upregulation of angiogenic genes (Vegf, Pecam1), increased vascularity(11–13), and increased blood flow rates(14). Notably, inhibition of angiogenesis using TNP-470 or fumagillin nanoparticles results in significantly decreased bone formation(1), indicating that angiogenesis is a requirement for maximal woven bone formation.
Alternatively, cyclic mechanical loading applied at physiological strains for relatively few cycles is non-damaging, and stimulates the formation of lamellar bone, a process known as strain adaptive bone modeling(15). In particular, a single bout of non-damaging, cyclic loading of the rat forelimb results in significantly increased periosteal lamellar bone formation at the ulnar mid-diaphysis(11). In contrast to the woven bone scenario, lamellar bone formation has not been associated with increased vascularity, and pro-angiogenic genes are only modestly upregulated following loading(16). Whether angiogenesis is required for maximal loading-induced lamellar bone formation is not known.
The αvβ3 integrin has been shown to be a marker for blood vessels undergoing angiogenesis(17), and its expression diminishes as blood vessels mature(18). Although the role of the αvβ3 integrin in angiogenesis is complex(19), knockout models have been used to demonstrate that when αvβ3 integrin remains unligated, due to either endogenous or therapeutic antagonism, it can act to cause endothelial cell apoptosis by inducing so-called integrin-mediated death(20). Antagonists against the αvβ3 integrin have been used to effectively inhibit blood vessel formation in models of pathological angiogenesis(21–23), though clinical success has been limited(24).
The objective of this study was to determine the effect of antagonizing the αvβ3 integrin by repeatedly injecting targeted nanoparticles following damaging and non-damaging mechanical loading known to stimulate woven or lamellar bone formation. First, the expression of the β3 integrin subunit as well as the distribution of αvβ3 integrin targeted nanoparticles in loaded and non-loaded forelimbs was examined. Additionally, vascularity and bone formation were quantified in the first two weeks following loading in animals receiving nanoparticles or vehicle. Finally, whole-bone and microindentation mechanical testing were used to quantify the biomechanical consequences of angiogenic inhibition. The results of this work clarify the role of angiogenesis in bone formation induced by mechanical loading.
2. METHODS & MATERIALS
2.1 Mechanical Loading
Male Fischer (F344/NHsd) rats were obtained at 13–14 weeks of age (Harlan) and housed under standard conditions until use in experiments at 18–22 weeks of age. The right forelimb of each animal was mechanically loaded using one of two loading protocols designed to induce new bone formation at the midshaft of the ulna, while the non-loaded left forelimb of each animal was used as an internal control. First, rats were anesthetized with isoflurane gas (1–3%). Specially designed fixtures were used to secure the olecranon process and the flexed carpus. Mechanical loading was performed using a material testing system (Instron Electropuls 1000) to apply force and monitor displacement. The damaging loading protocol (referred to as WBF loading) creates fatigue damage at the mid-diaphysis of the ulna that stimulates abundant woven bone formation(10). For WBF loading, a 0.3 N compressive pre-load was applied followed by a cyclic haversine waveform of 18 N at 2 Hz until an increase in peak displacement equal to 65% of the displacement to complete fracture (1.3 mm, relative to the 10th cycle) was achieved(11,25). By contrast, the non-damaging loading protocol (referred to as LBF loading) stimulates lamellar bone formation at the ulnar mid-diaphysis without creating damage or decreasing bone strength(11). For LBF loading, a 0.3 N compressive pre-load was applied followed by a cyclic rest-inserted trapezoidal waveform with a peak force of 15 N at 0.1 Hz for 100 cycles(11). Following loading, rats were given an intramuscular injection of analgesic (0.05 mg/kg buprenorphine) and allowed unrestricted cage activity. All protocols were approved by the institution’s Animal Studies Committee.
2.2 αvβ3 Integrin Targeted Nanoparticles
αvβ3 integrin targeted nanoparticles were used to inhibit angiogenesis by antagonizing the αvβ3 integrin. After loading, each rat was given nanoparticles (1.5 mL/kg i.v.) or vehicle (sterile water) every other day until sacrifice; the first injection was two days after loading. In one set of animals, nanoparticles were administered until day 7, but animals were not sacrificed until day 14. The nanoparticles were directed to the αvβ3 integrin with a peptidomimetic vitronectin antagonist developed by Bristol-Myers Squibb Medical Imaging (U.S. patent 6,511,648 and related patents), as described previously(26). Except where noted, nanoparticles were comprised of 20% (v/v) perfluorooctylbromide (Exfluor Research Corporation) and 2.0% (w/v) of a surfactant co-mixture, 1.7% (w/v) glycerin, and water for the balance. For MR spectroscopy, perfluoro-15-crown-5-ether (Exfluor Research Corp.) was substituted for perfluoroocytylbromide to increase sensitivity of 19F detection(27). The surfactant commixture included 99 mol% highly purified egg yolk lecithin (Avanti Polar Lipids), and 0.1 mg/mL of the ανβ3 integrin antagonist conjugated to PEG2000-phosphatidyl-ethanolamine (Kereos, Inc.). The surfactant components were dissolved in chloroform/methanol and dried in a 50 °C vacuum oven overnight. Nanoparticles were modified to include a custom synthesized Alexafluor594-phosphatidylethanomine lipid conjugate (0.1 mg/mL), which was substituted into the surfactant mixture at the expense of lecithin on an equimolar basis.
2.3 Fluorescence Imaging
In vivo fluorescence imaging was used to quantify nanoparticle delivery 3 days after WBF loading. αvβ3 targeted Alexafluor 594 nanoparticles were injected 3–4 hours before imaging. Both forelimbs of each animal were shaved, and the mid-diaphyseal region was marked to indicate the region of interest. Following this, animals were placed supine in the imaging system (IVIS-50, Caliper Labs) and anesthetized using isoflurane gas (1–3%). Fluorescence images (excitation: 570 nm, emission: 620 nm) were collected from the designated region of interest. Fluorescence intensity (photons/second) was quantified during a 1 minute scan using Living Image software (Caliper Life Sciences). Following imaging, forelimbs were harvested, fixed for 16–24 hours, and embedded in poly-(methyl methacrylate). Thin sections were cut longitudinally and imaged using standard fluorescence microscopy to visualize nanoparticles present at the site of bone formation.
2.4 MR Spectroscopy
Ex vivo magnetic resonance (MR) spectroscopy was used to quantify relative nanoparticle delivery in WBF and LBF loaded limbs compared to non-loaded control limbs 7 days after loading. Animals were anesthetized for injections using ketamine-xylazine cocktail (130.4 mg/kg ketamine, 19.6 mg/kg xylazine) to avoid contaminating the forelimbs with residual fluorine. All animals were injected with αvβ3 integrin targeted nanoparticles prepared using perfluoro-15-crown-5-ether (Exfluor Research Corp.). Animals were sacrificed 2–3 hours after injection. Immediately following sacrifice, forelimbs were harvested and embedded in degassed agarose gel (1%) containing 0.1% sodium azide. MR spectroscopy was conducted using an Agilent 11.7T Direct Drive MRI with a custom dual-tuned 1H/19F RF coil. Each forelimb was scanned individually along with a 0.5 mL vial containing 1.42 M NaF as a calibration and chemical shift reference. 19F spectra of each forelimb were separately acquired in a 10 minute scan using the following parameters: TR = 50 ms, bandwidth = 30,000 Hz, 33.50 ms acquisition time, 1024 averages, 50 µs hard RF pulse centered midway between the NaF and nanoparticle resonances. To determine the chemical shift and calibrate the fluorine concentration, a phantom of diluted nanoparticles (1.2 mL of nanoparticles at 19F concentration of 1.95 M) was also scanned with the NaF reference vial.
2.5 MicroCT Imaging
Ex vivo micro computed tomography (µCT40, Scanco Medical AG) was used to quantify bone structure and density at the ulnar mid-diaphysis 7 and 14 days after WBF loading. The central 8 mm of each ulna was scanned separately at 45 kV and 177 µA with 200 msec integration time. The scan tube diameter was 16.4 mm, and medium resolution was used to obtain a 16 µm voxel size. Scan slices were acquired in the transverse plane by placing the forelimb parallel to the z-axis of the scanner. Hand drawn contours (sigma = 1.2, support = 2, lower/upper threshold = 330/1000) were used to manually segment bone with Scanco imaging software. Woven bone volume was calculated by subtracting the original cortical bone volume from the total bone volume in the entire scan. Because woven bone is absent from control limbs, woven bone volume measured by microCT is entirely new woven bone formation. A prior study showed excellent agreement between microCT and dynamic histomorphometric determinations of woven bone area(10). Woven bone BMD was calculating by analyzing only woven bone in the middle 20 slices of the woven bone extent.
2.6 Dynamic Histomorphometry
Lamellar bone formation was quantified using dynamic histomorphometry. Rats were given intraperitoneal injections of fluorescent bone formation markers 3 days (calcein green - 5 mg/kg, Sigma) and 8 days after loading (alizarin complexone - 30 mg/kg, Sigma). Two days later, forelimbs were harvested and fixed as above. After 16–24 hours fixation, forelimbs were embedded in poly-(methyl methacrylate). 100 µm transverse sections were cut (SP 1600, Leica Microsystems) 1 mm distal to the midpoint, then polished to 30 µm and mounted on glass slides. Digital images of these sections were captured using fluorescence microscopy (Olympus IX-51) with fluorescein isothiocyanate (FITC) and tetramethylrhodamine isothiocyanate (TRITC) filters for calcein and alizarin, respectively. Images were analyzed in Bioquant OSTEO for periosteal bone formation rate (BFR/BS), mineral apposition rate (MAR), and mineralizing surface (MS/BS) as defined by the ASBMR committee for histomorphometry nomenclature(28). Endosteal parameters were not measured, due to the frequent absence of marrow cavity in the rat ulna.
2.7 Immunohistochemistry
Immunohistochemistry was used to quantify blood vessel number as well as visualize expression of β3 integrin. Histological analysis was performed on 5 µm thick cross sections of formalin-fixed, paraffin-embedded forelimbs cut 1 mm distal to the midpoint. After deparaffination in xylenes and rehydration in graded ethanol solutions, antigen retrieval was performed by overnight incubation in 0.33 M boric acid (B6867, Sigma) at 55 °C. Sections were first incubated for 20 minutes in 3% H2O2 to block endogenous peroxidase activity, then in normal goat serum (sc-2043, Santa Cruz – 1.5% in PBS) to reduce nonspecific background staining. Following this, slides were incubated in primary antibody (rabbit polyclonal) against von Willebrand Factor (vWF; AB7356, Millipore – 1:200) or β3 integrin (sc-14009, Santa Cruz – 1:50) diluted in 1.5% normal goat serum in PBS at 4 °C overnight. To visualize binding, biotinylated goat anti-rabbit (sc-2018, Santa Cruz) secondary antibody was applied for 30 minutes followed by avidin-biotin-peroxidase complex for 30 minutes. Finally, slides were developed using diaminobenzidine. The slides were then dehydrated, mounted, and imaged with bright field microscopy (20× objective). There was essentially no stain on negative control slides prepared by omitting the primary antibody. Stitched cross-sectional images were analyzed using FIJI(29).
2.8 Mechanical Testing
Mechanical testing of the rat forelimb was used to quantify the strength of the skeletal repair 7 and 14 days after WBF loading. Immediately following sacrifice, the intact forelimb of each animal was placed into the original loading fixtures. After a preload of 0.3 N, the forelimb was compressed axially in displacement control at 0.1 mm/s until fracture. Both loaded (right) and non-loaded (left) forelimbs were tested. From force-displacement data, the following outcomes were determined: ultimate force, stiffness, displacement to failure, pre-yield displacement, post-yield displacement, energy to failure, pre-yield energy, and post-yield energy. Immediately after this procedure, ulnae were dissected and placed into a PBS bath for microindentation testing (Biodent, Active Life Scientific)(30). A 90° conospherical probe with < 5 µm radius point (BP2 probe assembly) was used to indent the bone immediately adjacent to the fracture site created during axial compression. For loaded limbs, this site included woven bone induced from WBF loading, whereas non-loaded bones were indented in original cortical bone. A protocol of 4 N at 2 Hz for 5 cycles was used to acquire the force-displacement data from which total indentation distance (TID) and indentation distance increase (IDI) were determined.
2.9 Statistics
Results are given as fold changes (loaded limb / non-loaded limb) and plotted as mean ± standard deviation. Two-way analysis of variance (ANOVA) was used to compare across treatment groups and time points. Differences between individual treatment groups and time points were assessed using Fisher’s protected least significant difference test, while differences between loaded and non-loaded limbs were assessed using paired t-tests. In each case, p < 0.05 was considered significant.
3. RESULTS
3.1 β3 Expression and Nanoparticle Delivery at Ulnar Mid-Diaphysis after Damaging Loading
To provide rationale for the αvβ3 integrin targeting strategy, expression of the β3 integrin subunit was assessed by immunohistochemistry following damaging (WBF) mechanical loading (Figure 1). On days 1 and 3, few vessels in the expanded periosteal region expressed β3 integrin. By day 7, the neovasculature in the nascent woven bone strongly expressed β3 integrin. Expression at day 14 was intermediate, with mixed expression on vessels in the newly formed woven bone. These results demonstrate that the β3 integrin is primarily expressed on neovasculature, not mature vessels, and expression of the β3 integrin peaks at day 7 following WBF loading.
Figure 1. β3 Positive Vessels are Present in New Woven Bone Following WBF Loading.
Following damaging mechanical loading, immunohistochemistry revealed that β3 integrin was expressed on few vessels on A) day 1 and B) day 3. The strongest expression of β3 integrin occurs in neovasculature on C) day 7, though expression persists at D) day 14. Muscle (M), pre-existing cortical bone (CB), and new woven bone (WB) are labeled in each cross section, and the double sided arrow labels the expanded periosteal region (days 1 and 3) that becomes woven bone (days 7 and 14).
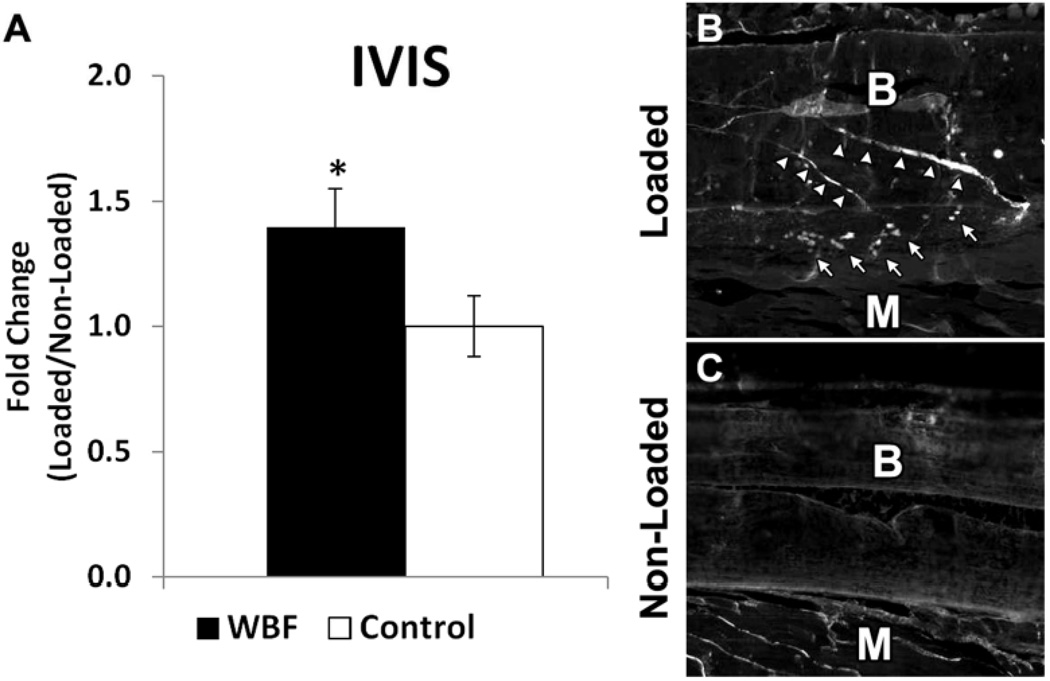
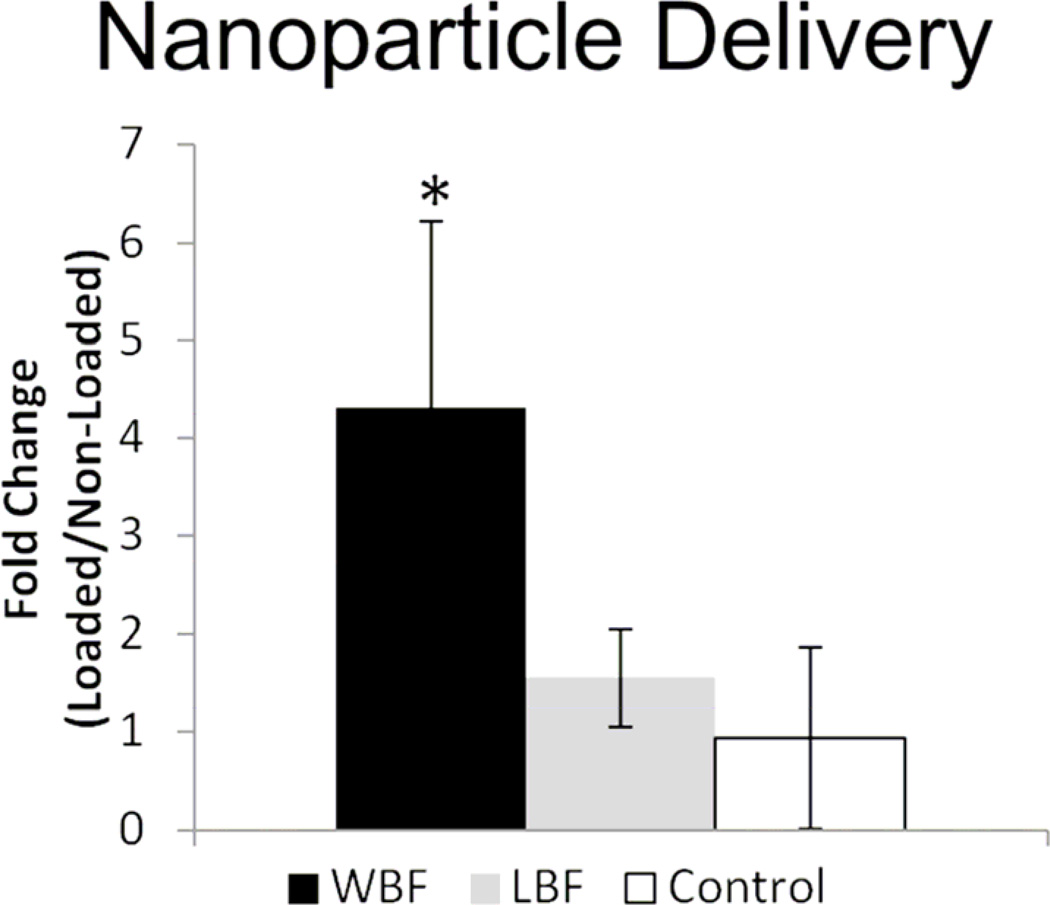
Several approaches were used to demonstrate the targeting of αvβ3 integrin targeted nanoparticles to limbs subjected to damaging loading compared to non-loaded limbs. First, in vivo imaging (IVIS) revealed a 40% increase in fluorescence at day 3 in WBF loaded limbs compared to non-loaded control limbs (Figure 2). Using post mortem fluorescence microscopy, nanoparticles were detected in loaded limbs at the sites of bone damage and periosteal expansion, but were not detected in non-loaded limbs. Next, limbs from animals injected with nanoparticles were harvested and subjected to MR spectroscopy. With increased signal-to-noise compared to IVIS imaging, MR spectroscopy detected a 4-fold increase in nanoparticle delivery at day 7 in WBF loaded limbs compared to non-loaded limbs (Figure 3). Moreover, the 19F signal did not differ between limbs subjected to non-damaging loading (LBF) and non-loaded limbs.
Figure 2. Fluorescent Microscopy and IVIS Imaging Reveals Nanoparticles Are Targeted to WBF Loaded Limbs.
αvβ3 integrin targeted nanoparticles were administered 3 days after WBF loading. A) Using IVIS in vivo imaging, WBF loaded limbs had approximately 40% increased fluorescence compared to control animals without nanoparticles. B) Loaded limbs had clear evidence of nanoparticles (arrows) near the site of bone damage (arrowheads), whereas C) non-loaded limbs had no evidence of nanoparticles. Muscle (M) and bone (B) are labeled in each longitudinal section. * p < 0.05 vs. Control.
Figure 3. MR Spectroscopy Demonstrates Increased Nanoparticle Delivery in WBF Loaded Limbs.
Using MR spectroscopy, nanoparticle delivery was quantified in WBF and LBF loaded limbs. WBF loaded limbs had a 4-fold increase in nanoparticle delivery compared to control animals without nanoparticles, whereas LBF loaded limbs did not differ significantly from control. * p < 0.05 vs. Control.
3.2 αvβ3 Integrin Antagonism by Targeted Nanoparticles Blunts Angiogenesis following Damaging and Non-Damaging Loading
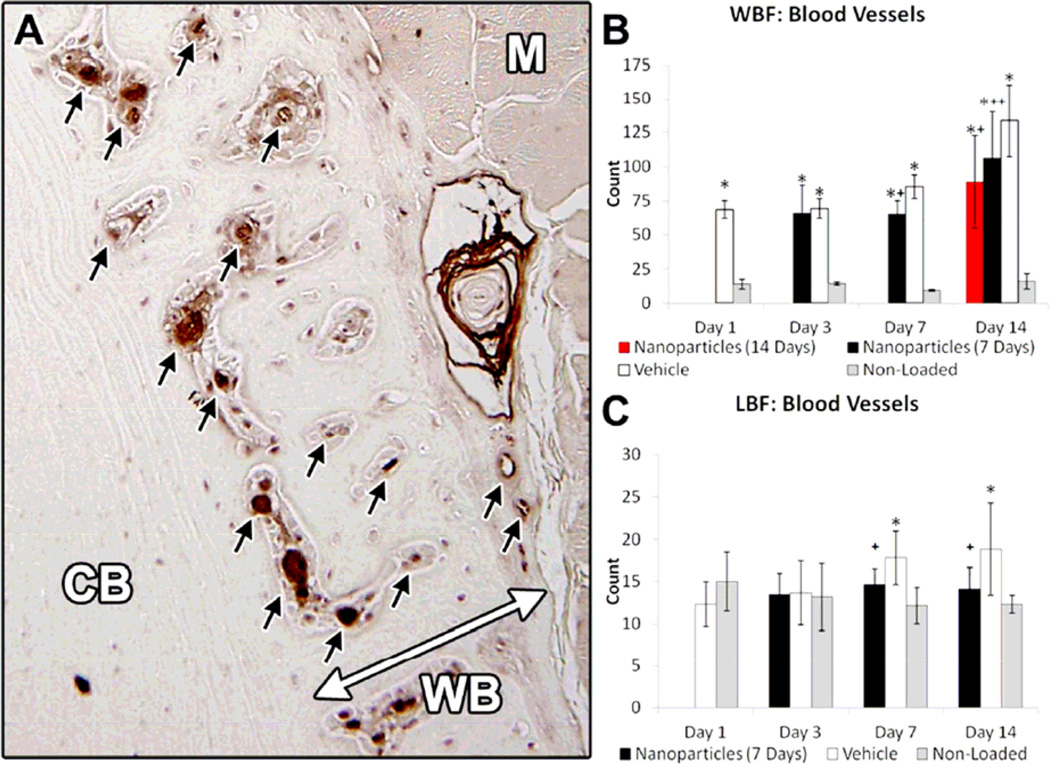
Both the large increases in vascularity induced by damaging (WBF) loading as well as the smaller increases in vascularity induced by non-damaging (LBF) loading were diminished by treatment with αvβ3 integrin targeted nanoparticles (Figure 4). From vWF immunohistochemistry, there was a significant increase in vascularity in loaded limbs compared to non-loaded limbs on days 1 and 3 after WBF loading. Nanoparticle treatment did not affect vascularity at these early time points. On day 7, vascularity was further increased in WBF loaded limbs compared to non-loaded limbs, and this increase was significantly inhibited by nanoparticle treatment. This treatment effect persisted on day 14, especially in WBF loaded animals that received nanoparticle treatment for the entire 14 days. By contrast, LBF loading was not associated with increased vascularity in loaded limbs compared to non-loaded limbs at days 1 or 3. However, there was a small (+50%) but significant increase in vascularity at days 7 and 14 that was effectively blocked by nanoparticle treatment.
Figure 4. Increase in Vasculature following Mechanical Loading Is Blunted by Nanoparticle Treatment.
A) Blood vessels (black arrows) were quantified using immunohistochemistry against von Willebrand Factor (vWF). Muscle (M), pre-existing cortical bone (CB), and new woven bone (WB) are labeled, and the double-sided arrow is the expanded periosteal region. B) Significant increases in WBF loaded limbs were evident at all time points with treatment-related effects at days 7 and 14 after loading. C) Small but significant increases in vascularity in LBF loaded limbs were inhibited using anti-angiogenic nanoparticles at days 7 and 14. * p < 0.05 vs. Non-Loaded. + p < 0.05 vs. Vehicle.
3.3 Decreased Angiogenesis Impairs Woven but Not Lamellar Bone Formation
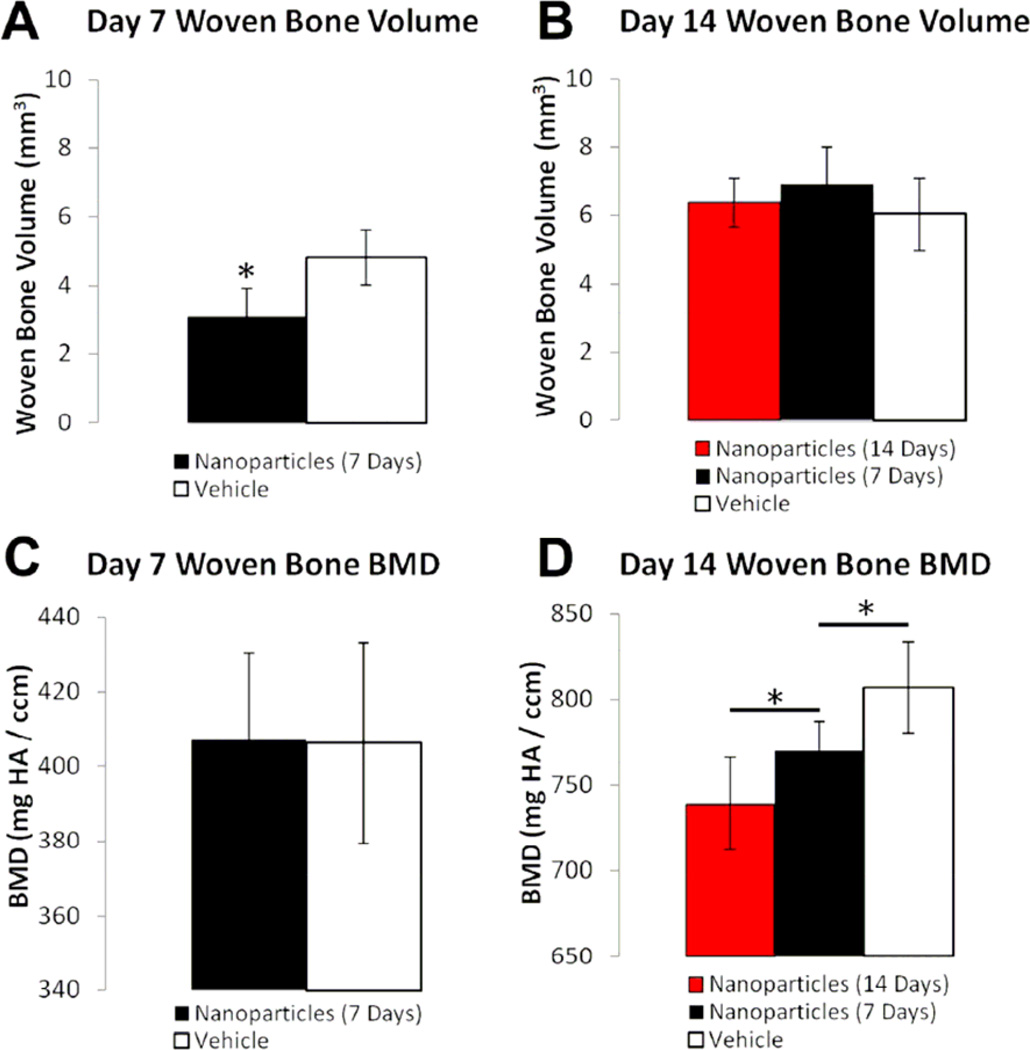
Inhibition of angiogenesis by nanoparticle treatment impaired woven bone formation after damaging (WBF) loading. On day 7, microCT imaging revealed an abundant woven bone formation response following WBF loading (Figure 5). Nanoparticle treatment significantly decreased (−36%) woven bone volume compared to vehicle treatment at this time point. The ability of nanoparticle treatment to blunt woven bone formation was transient, as microCT analysis on day 14 showed no difference in woven bone volume between nanoparticle and vehicle treated groups, regardless of duration of treatment. Nanoparticle treatment also affected woven bone BMD, but with the opposite temporal pattern as woven bone volume. Woven bone BMD at day 7 was not affected by nanoparticle treatment, whereas woven bone BMD at day 14 was significantly decreased by 7 days (−5%) and 14 days (−8%) of nanoparticle treatment.
Figure 5. Woven Bone Formation Is Impaired by Anti-Angiogenic Nanoparticles.
Woven bone volume and BMD were quantified using microCT measurements at day 7 and 14 following WBF loading. A) At day 7, nanoparticle treatment resulted in significantly less woven bone volume. B) At day 14, nanoparticle treatment for either 1 or 2 weeks did not result in any significant differences in woven bone volume. C) At day 7, there was no significant difference in woven bone BMD between nanoparticle and vehicle treated groups. D) At day 14, there was a significant and dose-dependent difference in woven bone BMD between nanoparticle and vehicle treated groups. * p < 0.05 vs. Vehicle.
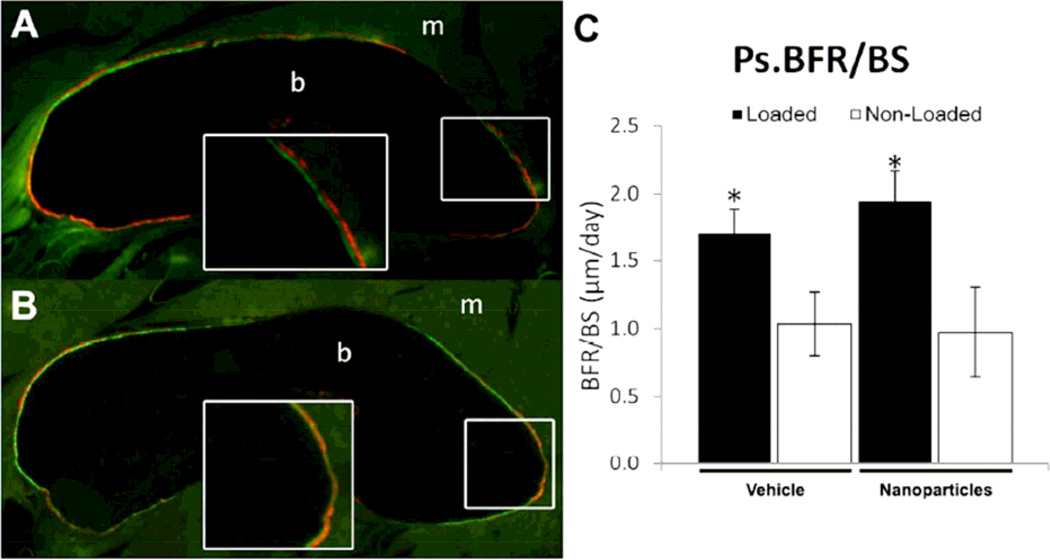
In contrast to the effect on woven bone formation, nanoparticle treatment did not impair lamellar bone formation following non-damaging (LBF) loading. Similar to previous work(11), periosteal bone formation was increased in loaded limbs compared to non-loaded controls (Figure 6), with significant increases in MS/BS (+35%) and BFR/BS (+64%). However, there was no significant effect due to nanoparticle treatment on periosteal MS/BS, MAR, or BFR/BS.
Figure 6. Lamellar Bone Formation Not Affected by Anti-Angiogenic Nanoparticles.
Calcein and alizarin complexone were administered at day 3 and day 8 following LBF loading. A) Loaded limbs had significantly increased lamellar bone formation, with obvious double labeling of the periosteal region (inset), compared to B) non-loaded limbs. C) Ps.BFR/BS was significantly elevated in loaded limbs compared to non-loaded limbs, but there were no significant differences in any measured parameter between nanoparticle and vehicle treated limbs. * p < 0.05 vs. Non-Loaded.
3.4 Vascularization of Woven Bone Affects the Structural Properties of Skeletal Repair
Because of the significant effect of nanoparticle treatment on vascularity, woven bone volume, and woven bone BMD, the mechanical integrity of the stress fracture repair was investigated 7 and 14 days after damaging (WBF) loading. At day 7, the loaded limbs were still recovering from the bone damage inflicted during WBF loading. Loaded limbs from vehicle treated animals had significantly decreased ultimate force and stiffness (measured by axial compression), and significantly increased IDI and TID (measured by microindentation) compared to non-loaded limbs (Table 1). At day 14, the loaded limbs from vehicle treated animals had largely recovered their mechanical function, with similar or improved strength compared to non-loaded limbs, as well as significant increases in pre-yield, post-yield, and total displacement and energy to failure. However, loaded limbs still exhibited significantly greater IDI and TID compared to non-loaded controls, indicating the diminished properties of woven bone relative to normal cortical bone.
Table 1.
Mechanical testing of stress fracture repair following WBF loading and treatment with αvβ3 integrin targeted nanoparticles or vehicle at 7 and 14 days. In the Day 14 Nanoparticles (7 days) group, animals were sacrificed at day 14, but only received nanoparticles for the first 7 days.
| Group | Forelimb | Ultimate Force (N) |
Stiffness (N/mm) |
Displ. to Failure (mm) |
Pre-Yield Displ. (mm) |
Post-Yield Displ. (mm) |
Energy to Failure (J) |
Pre-Yield Energy (J) |
Post-Yield Energy (J) |
IDI (mm) | TID (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 Vehicle | Loaded | 23.3 ± 2.4* | 8.9 ± 0.6* | 5.2 ± 1.0 | 3.5 ± 0.3 | 1.7 ± 0.8 | 72.5 ± 16.0 | 39.6 ± 4.4 | 32.9 ± 15.9 | 15.2 ± 1.8* | 131.0 ± 18.8* |
| Non-Loaded | 26.5 ± 1.4 | 11.6 ± 1.0 | 4.9 ± 0.5 | 3.4 ± 0.4 | 1.3 ± 0.4 | 72.8 ± 9.4 | 39.6 ± 2.8 | 31.8 ± 9.2 | 9.1 ± 0.9 | 58.3 ± 4.3 | |
| Day 7 Nanoparticles | Loaded | 22.7 ± 1.0* | 8.3 ± 0.4* | 4.8 ± 0.3 | 3.5 ± 0.3 | 1.3 ± 0.3* | 67.0 ± 5.4* | 40.0 ± 2.5 | 27.0 ± 6.1* | 18.2 ± 2.9* | 156.6 ± 20.8* |
| Non-Loaded | 25.6 ± 1.6 | 11.3 ± 0.5 | 5.0 ± 0.3 | 3.2 ± 0.3 | 1.8 ± 0.3 | 79.9 ± 5.3 | 39.7 ± 3.6 | 40.2 ± 4.6 | 10.8 ± 1.0 | 65.8 ± 6.4 | |
| Day 14 Vehicle | Loaded | 25.5 ± 2.2 | 10.7 ± 0.8 | 5.8 ± 0.4* | 3.3 ± 0.1* | 2.5 ± 0.5* | 98.2 ± 8.8* | 44.5 ± 4.1* | 53.7 ± 7.9* | 11.8 ± 1.6* | 87.6 ± 8.5* |
| Non-Loaded | 23.7 ± 2.4 | 11.2 ± 0.6 | 4.6 ± 0.8 | 2.9 ± 0.2 | 1.7 ± 0.9 | 69.0 ± 11.8 | 34.1 ± 3.4 | 34.9 ± 12.1 | 8.4 ± 0.8 | 60.4 ± 13.9 | |
| Day 14 Nanoparticles (7 days) | Loaded | 24.0 ± 1.2 | 11.5 ± 0.6* | 4.7 ± 0.8 | 3.0 ± 0.2* | 1.7 ± 0.7 | 75.6 ± 15.4 | 39.9 ± 3.3* | 35.7 ± 14.4 | 12.5 ± 1.0* | 97.4 ± 12.8* |
| Non-Loaded | 23.7 ± 1.8 | 12.9 ± 1.2 | 4.3 ± 0.8 | 2.4 ± 0.2 | 1.9 ± 0.7 | 67.9 ± 12.6 | 30.9 ± 2.4 | 37.0 ± 12.6 | 7.5 ± 2.0 | 56.6 ± 5.8 | |
| Day 14 Nanoparticles (14 days) | Loaded | 24.9 ± 1.1 | 13.4 ± 2.3 | 4.1 ± 0.9 | 2.8 ± 0.4* | 1.4 ± 0.6 | 70.7 ± 14.8 | 39.4 ± 4.0* | 31.3 ± 13.7 | 12.1 ± 1.6* | 94.7 ± 11.6* |
| Non-Loaded | 24.3 ± 1.9 | 14.6 ± 3.2 | 4.2 ± 0.6 | 2.3 ± 0.4 | 1.9 ± 0.6 | 68.5 ± 10.5 | 29.5 ± 2.9 | 39.0 ± 10.9 | 7.7 ± 2.0 | 53.6 ± 9.1 |
p < 0.05.
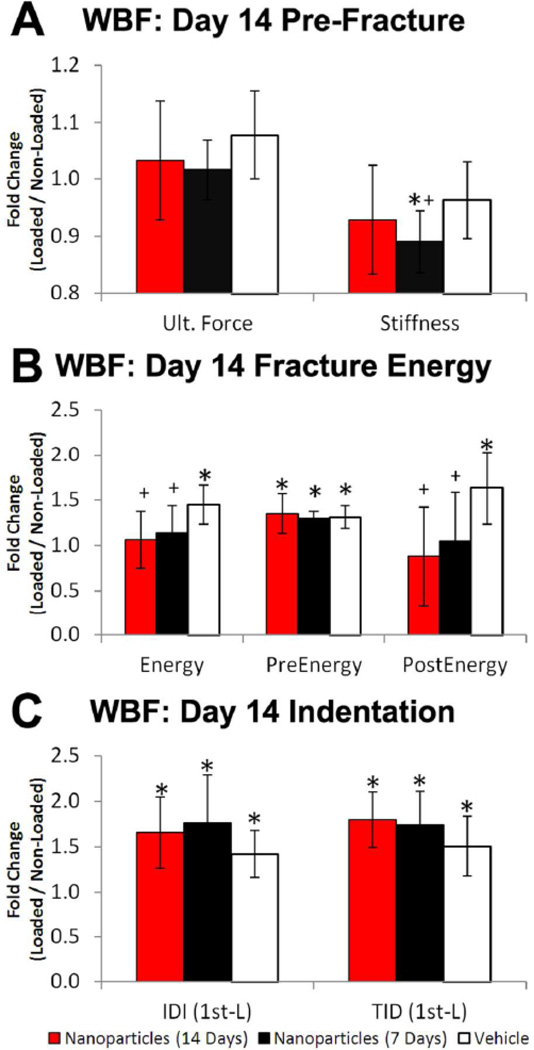
Treatment with αvβ3 integrin targeted nanoparticles impaired the recovery of mechanical properties following WBF loading (Figure 7). At day 14, loaded limbs from animals treated for 1 week with nanoparticles were mechanically inferior to loaded limbs from vehicle animals, with significant differences in ultimate force (−6%), stiffness (−8%), pre-yield displacement (−9%), post-yield displacement (−37%), post-yield energy (−36%), energy to failure (−21%), and IDI (+25%). These trends were exacerbated in animals treated for the full 2 weeks with nanoparticles, with significant differences in pre-yield displacement (+7%), post-yield displacement (−50%), displacement to failure (−21%), post-yield energy (−46%), energy to failure (−27%), and TID (+19%).
Figure 7. Nanoparticle Treatment Impairs Recovery of Mechanical Properties.
Mechanical properties of the skeletal repair following WBF loading were quantified using axial compression. A) Modest decreases in pre-fracture mechanical properties were observed in nanoparticle treated groups compared to vehicle groups. B) Fracture energy was significantly decreased in nanoparticle treated groups. While pre-yield energy was not different between nanoparticle and vehicle treated groups, both post-yield energy and total energy to failure were significantly lowered by anti-angiogenic treatment. C) Indentation testing illustrated greater indentation distance increase (IDI) and total indentation distance (TID) in loaded compared to non-loaded controls, indicating less resistance to indentation and crack propagation in the woven bone of loaded limbs compared to normal cortical bone. Nanoparticle treatment caused a trend of increased IDI and TID compared to vehicle treatment, indicating an effect on local woven bone properties. * p < 0.05 vs. Non-Loaded. + p < 0.05 vs. Vehicle.
4. DISCUSSION
A critical role for angiogenesis is well established in endochondral bone formation during development(7,8) and fracture healing(5,6). In this study, we examined the role of angiogenesis in physiological, non-endochondral bone formation scenarios, ranging from stress fracture repair to strain adaptive bone modeling. Adult rats were subjected to either damaging loading that led to woven bone formation (WBF) or non-damaging loading that stimulated lamellar bone formation (LBF), then injected with αvβ3 integrin targeted nanoparticles or vehicle. Using IVIS, fluorescence microscopy, and MR spectroscopy, nanoparticles were shown to be more highly concentrated in forelimbs subjected to WBF loading compared to LBF loaded or non-loaded controls. Nanoparticle treatment led to significant decreases in vascularity in both WBF and LBF loaded limbs at days 7 and 14. Decreased vascularity was associated with impaired woven bone formation following WBF loading, but did not affect the loading-induced increase in lamellar bone formation after LBF loading. In particular, nanoparticle treatment led to decreased woven bone volume at day 7 and decreased woven bone density at day 14. These decreases in woven bone formation were associated with inferior mechanical properties at day 7 and 14, particularly in post-yield behavior, indicating that angiogenesis is required for recovery of bone mechanical function after stress fracture. The cellular and molecular mechanisms though which angiogenesis regulates woven bone formation after damaging mechanical loading are still being discovered, and more study will be required to fully characterize this phenomenon.
The αvβ3 integrin has an important, complex role in angiogenesis. As a result, this integrin has been studied in a variety of models of physiological and pathological angiogenesis(19). This work is the first to investigate its expression in osteogenic mechanical loading. Following WBF loading, the β3 integrin subunit had limited expression on vasculature at days 1 and 3, but abundant expression on vessels located in large pores in the newly formed woven bone at days 7 and 14. These results are consistent with the hypothesis that angiogenesis begins near day 3 during stress fracture healing(1), and echo wound healing reports that demonstrated the expression of αvβ3 receptor in new vessels, but not mature vessels(18). The robust expression of β3 integrin observed in the vasculature as late as day 14 was unexpected, and suggests that vascular remodeling continues as the woven bone’s density increases from day 7 to 14(10). The sequence of periosteal responses following stress fracture has been summarized in Table 2.
Table 2.
Summary of Periosteal Responses following Stress Fracture
| Days | Tissue Response | Reference |
|---|---|---|
| 0–3 | Local Vasodilation | Tomlinson 2013(57) |
| 1–7 | Periosteal Expansion | Silva 2006(58), Matsuzaki 2007(12), Wohl 2009(13) |
| 3–7 | Woven Bone Formation | Uthgenannt 2007(10), Silva 2006(58), Matsuzaki 2007(12), Wohl 2009(13) |
| 3–14 | Periosteal Angiogenesis | Tomlinson 2013(1), McKenzie 2011(11), Matsuzaki 2007(12), current study |
| 7–14 | Woven Bone Densification | Uthgenannt 2007(10) |
| 10–70 | Woven Bone Remodeling | Kidd 2010(53) |
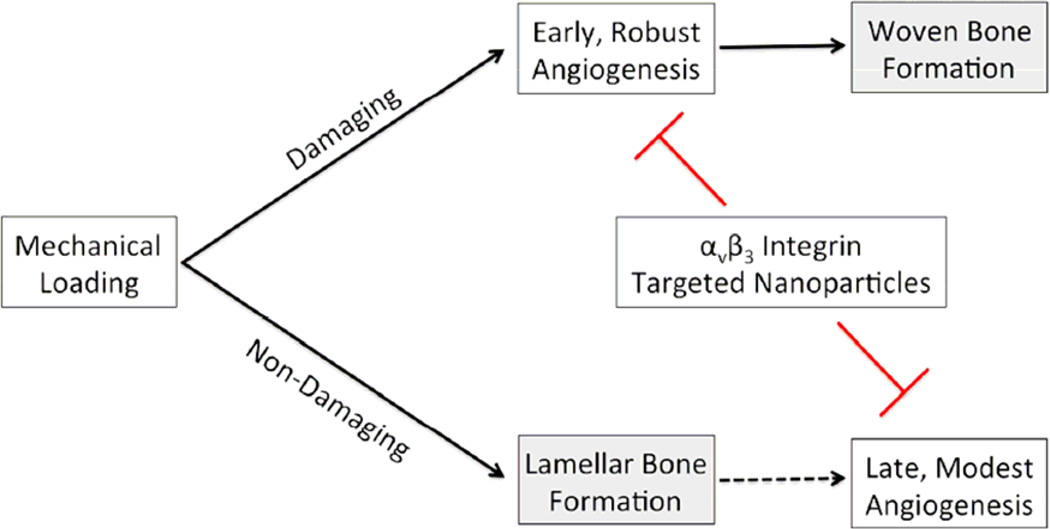
Both WBF and LBF loaded limbs have increased vascularity compared to control limbs at days 7 and 14, demonstrating that angiogenesis accompanies both types of bone formation induced by mechanical loading. This is the first report of increased vasculature in the context of loading-induced lamellar bone formation. However, the magnitude of the increase is markedly greater after WBF loading (+700% at day 14) than after LBF loading (+50% at day 14). While targeted nanoparticles inhibited angiogenesis following both WBF and LBF loading, decreased vascularity only impaired woven bone formation, not lamellar. This important result is illustrated in Figure 8. It is important to note that measures of vascularity were performed by counting vessels positive for von Willebrand Factor (vWF), a marker of differentiated endothelium(31). Previous studies have shown that new vessels may appear positive for αvβ3 integrin before expressing vWF(23), so blood vessel counts reported here are conservative, representing mature vessels.
Figure 8. Divergent Requirements of Angiogenesis for Woven and Lamellar Bone Formation.
Damaging mechanical loading generates a stress fracture in the bone, stimulating early and robust angiogenesis that leads to woven bone formation. Non-damaging mechanical loading directly stimulates lamellar bone formation (strain adaptive bone modeling) and is associated with late, modest angiogenesis. Both angiogenic responses are inhibited by αvβ3 integrin targeted nanoparticles, impairing woven bone formation without affecting lamellar bone formation. The angiogenesis that follows non-damaging mechanical loading may be driven by the accumulation of additional lamellar bone rather than mechanical loading, as indicated by the dashed line.
The increase in angiogenesis following both WBF and LBF loading was inhibited by nanoparticle treatment, but only woven bone formation was adversely affected. Interestingly, woven bone volume after WBF loading was diminished at day 7 by nanoparticle treatment, but not at day 14, despite continued nanoparticle treatment. On the other hand, woven bone BMD was unaffected by nanoparticle treatment at day 7, but was significantly decreased at day 14. In addition, woven bone BMD in animals treated for 14 days was less than animals only treated for the first 7 days. These results led us to hypothesize that impaired vascularity in skeletal repair has two distinct consequences. In this working model, an initial bottleneck to healing occurs in the first week, as diminished angiogenesis delays the rapid expansion of subperiosteal osteoprogenitors, resulting in reduced woven bone volume. However, sufficient vasculature is present to erase the deficit within two weeks. A later bottleneck occurs in the repair process as decreased vascularity impedes bone mineralization. More study is required to confirm this hypothesis.
Results from this study indicate an important role for αvβ3 integrin mediated angiogenesis in non-endochondral bone formation. In a previous study, αvβ3 integrin targeted nanoparticles containing the anti-angiogenic mycotoxin fumagillin inhibited angiogenesis and woven bone formation 7 days after stress fracture in rats(1). Here, animals treated serially with αvβ3 integrin targeted nanoparticles without fumagillin had similar decreases in vascularity and woven bone volume. Although previous studies have demonstrated that fumagillin nanoparticles have a superior anti-angiogenic effect compared to nanoparticles without fumagillin in multiple animal models(32), the effect of receptor antagonism with repeatedly dosed αvβ3 integrin targeted nanoparticles was sufficient to inhibit angiogenesis following stress fracture in this study. However, angiogenesis involves a complicated crosstalk between growth factors, receptors, and integrins (including the αvβ3 integrin as well as others)(33). In conjunction with angiogenesis modulated by the αvβ3 integrin, both VEGF and FGF pro-angiogenic signaling pathways appear active following damaging mechanical loading(11,16,34) and are known to play important roles in other osteogenic scenarios(4,35–38). In this study, the anti-angiogenic effect of the targeted nanoparticles was incomplete; following WBF loading, vascularity was increased at day 14 compared to day 3 regardless of treatment duration (Figure 4B). Therefore, these results point to a working hypothesis that multiple complementary angiogenic pathways may contribute independently to the vascularization of woven bone following stress fracture.
The mechanical consequences of poor skeletal repair due to inadequate vascularization are not well understood. Biomechanical testing of forelimbs with healing stress fractures revealed significant decreases in mechanical integrity associated with αvβ3 integrin targeted nanoparticle treatment. Although pre-fracture properties (ultimate force, stiffness) were mostly unaffected by treatment, properties associated with resistance to fracture, such as post-yield deformation, were severely affected. Thus, recovery of bone’s fracture resistance after injury may proceed more slowly than expected in patient populations with poor vascular function, creating an additional risk of reinjury. This finding has important clinical implications, especially since anti-angiogenic therapy is a widespread, FDA approved treatment for many cancers(39,40), and fracture risk in cancer patients is known to be elevated(41,42). In addition, pathologies associated with decreased skeletal vascularization, such as osteoporosis(43,44), diabetes(45,46), and smoking(47), may be affected in the same way.
A novel result from this study is that loading-induced lamellar bone formation is associated with new vessel formation, but is not dependent on angiogenesis. In previous studies, it was revealed that lamellar bone formation following mechanical loading is associated with the modest upregulation of some pro-angiogenic genes (Vegf, Hif1α), but no increases in vascularity at day 3(11). Here, these results were clarified. First, lamellar bone formation is associated with significantly increased vascularity at days 7 and 14, confirming that angiogenesis is coupled to osteogenesis, even during relatively modest bone apposition. Treatment with αvβ3 integrin targeted nanoparticles inhibited this increase in vascularity, but did not affect the rate of lamellar bone formation. These results suggest that the vasculature already present at the ulnar mid-diaphysis is sufficient for lamellar bone formation, with subsequent angiogenesis driven by the increase in bone volume. Furthermore, this data leads to the hypothesis that the pro-angiogenic factors previously associated with the early stages of lamellar bone formation may be acting in non-angiogenic roles – HIF-1α has been implicated as an inhibitor of loading-induced bone formation(48), and VEGF is known to have many splice isoforms with a variety of functions(49,50). More study is required to understand how these factors may influence strain adaptive bone modeling.
The conclusion that decreased vascularity leads to decreased woven bone formation and delayed recovery of mechanical properties is dependent on the assumption that αvβ3 integrin targeted nanoparticles do not directly affect bone cells. While the αvβ3 integrin is not expressed on osteoblasts or osteocytes, it is critical for osteoclast function(51). In previous studies of ulnar stress fracture in rats, osteoclasts were not detected until 10–14 days after loading(52–54), suggesting that osteoclast activity is not significant until after the angiogenic and woven bone responses are well underway. Additionally, the nanoparticles used in this study are constrained to the vasculature due to their size(55), so interaction between nanoparticles and osteoclasts is unlikely. Therefore, the decreases in woven bone formation and density are attributed to decreased vascularity, not a direct effect on bone cells. Regarding lamellar bone formation, there is no evidence that osteoclasts play a role in periosteal strain adaptive bone modeling in adult rodents(56), and periosteal osteoclasts were not observed in control or LBF loaded ulnae. Moreover, the lack of effect of nanoparticles on lamellar bone formation indicates that bone cell function was not impaired by the treatment.
5. CONCLUSION
In this study, animals were subjected to either damaging (WBF) or non-damaging (LBF) osteogenic mechanical loading, then injected with αvβ3 integrin targeted nanoparticles or vehicle. The expression of the β3 integrin subunit is transient following WBF loading, with maximal expression on vessels in newly formed woven bone at day 7. Accordingly, nanoparticle delivery was found to be significantly increased in WBF loaded limbs compared to non-loaded limbs at both days 3 and 7. αvβ3 integrin targeted nanoparticles inhibited the increased vascularity after WBF and LBF loading, indicating a critical role for the αvβ3 integrin in angiogenesis following osteogenic mechanical loading. Decreased vascularity led to impaired woven bone formation and decreased mechanical properties following WBF loading, but did not affect lamellar bone formation after LBF loading. Importantly, these results demonstrate that impaired angiogenesis negatively affects the skeletal repair process following damaging mechanical loading. By contrast, impaired vascular function does not appear to affect strain adaptive bone modeling. More study is required to identify the cellular and molecular mechanisms regulating angiogenesis and osteogenesis following mechanical loading.
Acknowledgements
This study was funded by a grant from the National Institutes of Health (NIH R01 AR050211) and was performed at a facility supported by the Washington University Musculoskeletal Research Center (NIH P30 AR057235). Nanomedicine research support was received from the NIH (HL113392, HL1122518, CA100623, CA154737, HL094470, AR056468, NS073457, CA136398) and the American Heart Association (0835426N and 11IRG5690011). The authors would like to thank Ralph W. Fuhrhop for the preparation of targeted nanoparticles.
Dr. Lanza is a scientific cofounder of Kereos, Inc, St. Louis, which has licensed angiogenesis-targeted perfluorocarbon nanotechnology intellectual property from Washington University/Barnes-Jewish Hospital for clinical development.
Footnotes
Disclosures: All other authors state that they have no conflicts of interest.
Author Roles
Research Design: RET, AHS, JDQ, GML, MJS. Performed Research: RET, JDQ. Data Analysis: RET, JDQ, MJS. Drafting of Manuscript: RET, GML, MJS. Approving of Manuscript: RET, AHS, JDQ, GML, MJS.
Contributor Information
Anne H. Schmieder, Email: anne@cmrl.wustl.edu.
James D. Quirk, Email: quirkj@mir.wustl.edu.
Gregory M. Lanza, Email: greg.lanza@mac.com.
Matthew J. Silva, Email: silvam@wustl.edu.
References
- 1.Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone. 2013;52(1):212–219. doi: 10.1016/j.bone.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. Journal of Bone and Mineral Research. 2005;20(7):1114–1124. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Simpson AH, Kenwright J, Triffitt JT. Effect of lengthening rate on angiogenesis during distraction osteogenesis. Journal of Orthopaedic Research. 1999;17(3):362–367. doi: 10.1002/jor.1100170310. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. Journal of Bone and Mineral Research. 2008;23(5):596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 6.Glowacki J. Angiogenesis in fracture repair. Clinical Orthopaedics and Related Research. 1998 Oct;(355S):S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 7.Pechak DG, Kujawa MJ, Caplan AI. Morphological and histochemical events during first bone formation in embryonic chick limbs. Bone. 1986;7(6):441–458. doi: 10.1016/8756-3282(86)90004-9. [DOI] [PubMed] [Google Scholar]
- 8.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 9.Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. The Anatomical Record. 2001;262(4):398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 10.Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. Journal of Bone and Mineral Research. 2007;22(10):1548–1556. doi: 10.1359/jbmr.0070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie JA, Silva MJ. Comparing histological, vascular and molecular responses associated with woven and lamellar bone formation induced by mechanical loading in the rat ulna. Bone. 2011;48(2):250–258. doi: 10.1016/j.bone.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki H, Wohl GR, Novack DV, Lynch JA, Silva MJ. Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna. Calcified Tissue International. 2007;80(6):391–399. doi: 10.1007/s00223-007-9031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohl GR, Towler DA, Silva MJ. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone. 2009;44(2):320–330. doi: 10.1016/j.bone.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson RE, Silva MJ, Shoghi KI. Quantification of skeletal blood flow and fluoride metabolism in rats using PET in a pre-clinical stress fracture model. Molecular Imaging and Biology. 2012;14(3):348–354. doi: 10.1007/s11307-011-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone. 1991;12(2):73–79. doi: 10.1016/8756-3282(91)90003-2. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie JA, Bixby EC, Silva MJ. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PLoS ONE. 2011 doi: 10.1371/journal.pone.0029328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 18.Clark RA, Tonnesen MG, Gailit J, Cheresh DA. Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. The American Journal of Pathology. 1996;148(5):1407–1421. [PMC free article] [PubMed] [Google Scholar]
- 19.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Current Opinion in Cell Biology. 2008;20(5):514–519. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Cheresh DA, Stupack DG. Integrin-mediated death: an explanation of the integrin-knockout phenotype? Nature Medicine. 2002;8(3):193–194. doi: 10.1038/nm0302-193. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. The Journal of Clinical Investigation. 1995;96(4):1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(18):9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storgard CM, Stupack DG, Jonczyk A, Goodman SL, Fox RI, Cheresh DA. Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. The Journal of Clinical Investigation. 1999;103(1):47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stupp R, Ruegg C. Integrin inhibitors reaching the clinic. Journal of Clinical Oncology. 2007;25(13):1637–1638. doi: 10.1200/JCO.2006.09.8376. [DOI] [PubMed] [Google Scholar]
- 25.Uthgenannt BA, Silva MJ. Use of the rat forelimb compression model to create discrete levels of bone damage in vivo. Journal of Biomechanics. 2007;40(2):317–324. doi: 10.1016/j.jbiomech.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Wickline SA, Neubauer AM, Winter P, Caruthers S, Lanza G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(3):435–441. doi: 10.1161/01.ATV.0000201069.47550.8b. [DOI] [PubMed] [Google Scholar]
- 27.Morawski AM, Winter PM, Yu X, Fuhrhop RW, Scott MJ, Hockett F, Robertson JD, Gaffney PJ, Lanza GM, Wickline SA. Quantitative "magnetic resonance immunohistochemistry" with ligand-targeted (19)F nanoparticles. Magnetic Resonance in Medicine. 2004;52(6):1255–1262. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 28.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diez-Perez A, Guerri R, Nogues X, Caceres E, Pena MJ, Mellibovsky L, Randall C, Bridges D, Weaver JC, Proctor A, Brimer D, Koester KJ, Ritchie RO, Hansma PK. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. Journal of Bone and Mineral Research. 2010;25(8):1877–1885. doi: 10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han RN, Tanswell AK, Post M. Ontogeny of reactivity to endothelial cell markers during development of the embryonic and fetal rat lung. Histology and Histopathology. 1992;7(4):591–597. [PubMed] [Google Scholar]
- 32.Winter PM, Schmieder AH, Caruthers SD, Keene JL, Zhang H, Wickline SA, Lanza GM. Minute dosages of alpha(nu)beta3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. The FASEB Journal. 2008;22(8):2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapraeger AC, Ell BJ, Roy M, Li X, Morrison OR, Thomas GM, Beauvais DM. Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between alphaVbeta3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J. 2013;280(10):2194–2206. doi: 10.1111/febs.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez MD, Schmid GJ, McKenzie JA, Ornitz DM, Silva MJ. Healing of non-displaced fractures produced by fatigue loading of the mouse ulna. Bone. 2010;46(6):1604–1612. doi: 10.1016/j.bone.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Z, Lafage-Proust MH, Plouet J, Bloomfield S, Alexandre C, Vico L. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. Journal of Bone and Mineral Research. 2004;19(9):1471–1480. doi: 10.1359/JBMR.040517. [DOI] [PubMed] [Google Scholar]
- 36.Rundle CH, Miyakoshi N, Ramirez E, Wergedal JE, Lau KH, Baylink DJ. Expression of the fibroblast growth factor receptor genes in fracture repair. Clinical Orthopaedics and Related Research. 2002;(403):253–263. doi: 10.1097/00003086-200210000-00037. [DOI] [PubMed] [Google Scholar]
- 37.Schmid GJ, Kobayashi C, Sandell LJ, Ornitz DM. Fibroblast growth factor expression during skeletal fracture healing in mice. Developmental Dynamics. 2009;238(3):766–774. doi: 10.1002/dvdy.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima F, Ogasawara A, Goto K, Moriya H, Ninomiya Y, Einhorn TA, Yamazaki M. Spatial and temporal gene expression in chondrogenesis during fracture healing and the effects of basic fibroblast growth factor. Journal of Orthopaedic Research. 2001;19(5):935–944. doi: 10.1016/S0736-0266(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 39.Mayer RJ. Two steps forward in the treatment of colorectal cancer. The New England Journal of Medicine. 2004;350(23):2406–2408. doi: 10.1056/NEJMe048098. [DOI] [PubMed] [Google Scholar]
- 40.Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. The Keio Journal of Medicine. 2012;61(2):47–56. doi: 10.2302/kjm.61.47. [DOI] [PubMed] [Google Scholar]
- 41.Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11:384. doi: 10.1186/1471-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, Gass M, Leboff MS. Fracture risk among breast cancer survivors: results from the Women's Health Initiative Observational Study. Archives of Internal Medicine. 2005;165(5):552–558. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 43.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12(2):283–289. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 44.Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. The Journals of Gerontology. 2003;58(4):362–366. doi: 10.1093/gerona/58.4.m362. [DOI] [PubMed] [Google Scholar]
- 45.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22(9):1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 46.Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30(3):498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23(8):2081–2092. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 48.Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1alpha protein negatively regulates load-induced bone formation. The Journal of Biological Chemistry. 2011;286(52):44449–44456. doi: 10.1074/jbc.M111.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 50.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16(7):572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura I, Duong le T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. Journal of Bone and Mineral Metabolism. 2007;25(6):337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh YF, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. Journal of Orthopaedic Research. 2002;20(4):764–771. doi: 10.1016/S0736-0266(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 53.Kidd LJ, Stephens AS, Kuliwaba JS, Fazzalari NL, Wu AC, Forwood MR. Temporal pattern of gene expression and histology of stress fracture healing. Bone. 2010;46(2):369–378. doi: 10.1016/j.bone.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23(3):275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 55.Pan D, Pramanik M, Senpan A, Allen JS, Zhang H, Wickline SA, Wang LV, Lanza GM. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. The FASEB Journal. 2011;25(3):875–882. doi: 10.1096/fj.10-171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(4):319–338. doi: 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomlinson RE, Shoghi KI, Silva MJ. Nitric Oxide Mediated Vasodilation Increases Blood Flow During the Early Stages of Stress Fracture Healing. J Appl Physiol. 2013;(1985) doi: 10.1152/japplphysiol.00957.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva MJ, Uthgenannt BA, Rutlin JR, Wohl GR, Lewis JS, Welch MJ. In vivo skeletal imaging of 18F-fluoride with positron emission tomography reveals damage- and time-dependent responses to fatigue loading in the rat ulna. Bone. 2006;39(2):229–236. doi: 10.1016/j.bone.2006.01.149. [DOI] [PubMed] [Google Scholar]