Abstract
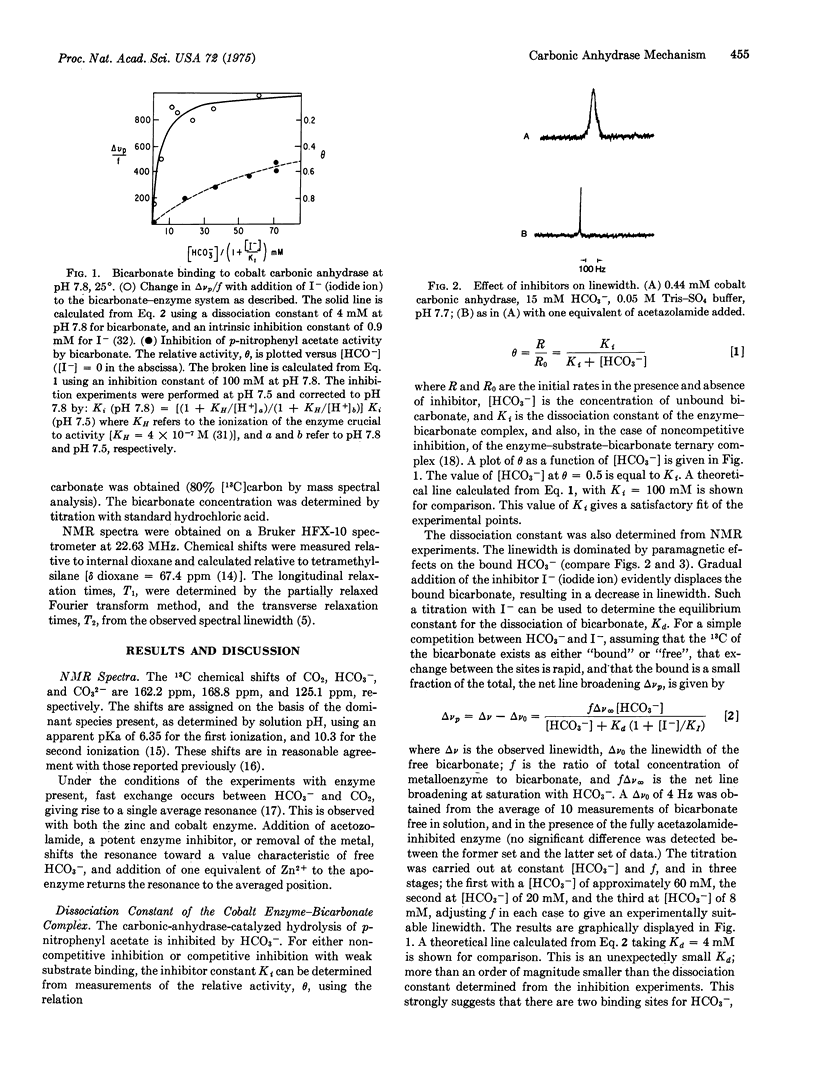
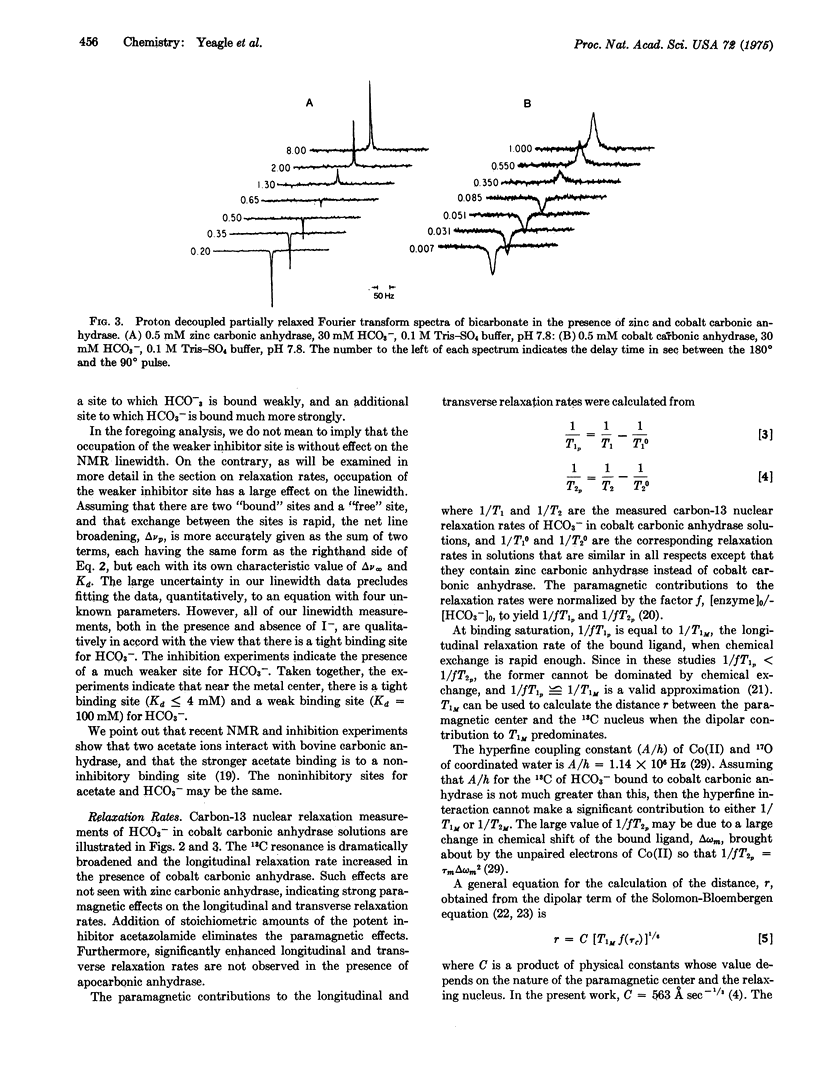
Binding of the substrate, bicarbonate, to bovine cobalt carbonic anhydrase (carbonate hydrolyase, EC 4.2.1.1) has been studied with 13-C nuclear magnetic resonance. Two binding sites for bicarbonate have been identified. One loosely binds bicarbonate, inhibits p-nitrophenyl acetate activity, and must be the bicarbonate substrate binding site; the other tightly binds bicarbonate, is noninhibitory, and plays another role. Spinlattice relaxation times for the carbon atom of bicarbonate indicate that the substrate bicarbonate is bound directly to the metal center of the enzyme, while the other bicarbonate is bound in the outer coordination sphere of the metal. It is proposed that dehydration proceeds via HCO-3 minus coordinated directly to the metal center, while the outer sphere bicarbonate facilitates catalytically important proton transfers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coleman J. E. Mechanism of action of carbonic anhydrase. Subtrate, sulfonamide, and anion binding. J Biol Chem. 1967 Nov 25;242(22):5212–5219. [PubMed] [Google Scholar]
- Fabry M. E., Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. IV. Proton relaxation at the active site of carbonic anhydrase. J Biol Chem. 1970 Sep 10;245(17):4256–4262. [PubMed] [Google Scholar]
- Fung C. H., Mildvan A. S., Allerhand A., Komoroski R., Scrutton M. C. Interaction of pyruvate with pyruvate carboxylase and pyruvate kinase as studied by paramagnetic effects on 13 C relaxation rates. Biochemistry. 1973 Feb;12(4):620–629. doi: 10.1021/bi00728a009. [DOI] [PubMed] [Google Scholar]
- Fung C. H., Mildvan A. S., Leigh J. S., Jr Electron and nuclear magnetic resonance studies of the interaction of pyruvate with transcarboxylase. Biochemistry. 1974 Mar 12;13(6):1160–1169. doi: 10.1021/bi00703a017. [DOI] [PubMed] [Google Scholar]
- Henkens R. W., Sturtevant J. M. Extrinsic Cotton effects in a metal chelator-bovine carbonic anhydrase complex. Biochemistry. 1972 Jan 18;11(2):206–210. doi: 10.1021/bi00752a010. [DOI] [PubMed] [Google Scholar]
- KERNOHAN J. C. THE PH-ACTIVITY CURVE OF BOVINE CARBONIC ANHYDRASE AND ITS RELATIONSHIP TO THE INHIBITION OF THE ENZYME BY ANIONS. Biochim Biophys Acta. 1965 Feb 22;96:304–317. [PubMed] [Google Scholar]
- Kandel M., Gornall A. G., Wong S. C., Kandel S. I. Some characteristics of human, bovine, and horse carbonic anhydrases as revealed by inactivation studies. J Biol Chem. 1970 May 10;245(9):2444–2450. [PubMed] [Google Scholar]
- Khalifah R. G. Carbon dioxide hydration activity of carbonic anhydrase: paradoxical consequences of the unusually rapid catalysis. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1986–1989. doi: 10.1073/pnas.70.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifah R. G., Edsall J. T. Carbon dioxide hydration activity of carbonic anhydrase: kinetics of alkylated anhydrases B and C from humans (metalloenzymes-isoenzymes-active sites-mechanism). Proc Natl Acad Sci U S A. 1972 Jan;69(1):172–176. doi: 10.1073/pnas.69.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSKOG S. Effects of pH and inhibitors on some properties related to metal binding in bovine carbonic anhydrase. J Biol Chem. 1963 Mar;238:945–951. [PubMed] [Google Scholar]
- LINDSKOG S., MALMSTROM B. G. Metal binding and catalytic activity in bovine carbonic anhydrase. J Biol Chem. 1962 Apr;237:1129–1137. [PubMed] [Google Scholar]
- Lanir A., Navon G. NMR studies of the two binding sites of acetate ions to manganese(II) carbonic anhydrase. Biochim Biophys Acta. 1974 Mar 21;341(1):75–84. doi: 10.1016/0005-2744(74)90067-9. [DOI] [PubMed] [Google Scholar]
- Lindskog S., Coleman J. E. The catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2505–2508. doi: 10.1073/pnas.70.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog S. Interaction of cobalt(II)--carbonic anhydrase with anions. Biochemistry. 1966 Aug;5(8):2641–2646. doi: 10.1021/bi00872a023. [DOI] [PubMed] [Google Scholar]
- Löchmuller C. H., Galbraith J., Walter R. L., Willis R. E. Metal-ion distribution in metalloproteins by proton-induced x-ray emission analysis. Anal Biochem. 1974 Feb;57(2):618–622. doi: 10.1016/0003-2697(74)90118-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Miziorko H. M., Mildvan A. S. Electron paramagnetic resonance, 1-H, and 13C nuclear magnetic resonance studies of the interaction of manganese and bicarbonate with ribulose 1, 5-diphosphate carboxylase. J Biol Chem. 1974 May 10;249(9):2743–2750. [PubMed] [Google Scholar]
- Morrow J. S., Keim P., Visscher R. B., Marshall R. C., Gurd F. R. Interaction of 13 CO 2 and bicarbonate with human hemoglobin preparations. Proc Natl Acad Sci U S A. 1973 May;70(5):1414–1418. doi: 10.1073/pnas.70.5.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYMAN P., LINDSKOG S. AMINO ACID COMPOSITION OF VARIOUS FORMS OF BOVINE AND HUMAN ERYTHROCYTE CARBONIC ANHYDRASE. Biochim Biophys Acta. 1964 Apr 6;85:141–151. doi: 10.1016/0926-6569(64)90174-9. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Guilbert L. J. Carbonic anhydrase catalyzed hydrolysis and decarboxylation. Kinetic studies of enzyme-catalyzed decomposition of mono- and disubstituted derivatives of carbonic acid. Biochemistry. 1974 Jan 1;13(1):70–78. doi: 10.1021/bi00698a012. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. VI. Kinetic studies of noncompetitive inhibition of enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1968 Aug;7(8):2936–2945. doi: 10.1021/bi00848a034. [DOI] [PubMed] [Google Scholar]
- Riepe M. E., Wang J. H. Infrared studies on the mechanism of action of carbonic anhydrase. J Biol Chem. 1968 May 25;243(10):2779–2787. [PubMed] [Google Scholar]
- Taylor P. W., Feeney J., Burgen A. S. Investigation of the mechanism of ligand binding with cobalt(II) human carbonic anhydrase by 1 H and 19 F nuclear magnetic resonance spectroscopy. Biochemistry. 1971 Oct 12;10(21):3866–3875. doi: 10.1021/bi00797a011. [DOI] [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. 3. Detection and properties of enzyme-metal-substrate and enzyme-metal-inhibitor bridge complexes with manganese(II) and iron(II). J Biol Chem. 1972 Jun 10;247(11):3454–3463. [PubMed] [Google Scholar]
- Wang J. H. Directional character of proton transfer in enzyme catalysis. Proc Natl Acad Sci U S A. 1970 Jul;66(3):874–881. doi: 10.1073/pnas.66.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. L., Nyman P. O., Malmström B. G. Inhibition and chemical modifications of human erythrocyte carbonic anhydrase B. J Biol Chem. 1967 Sep 25;242(18):4212–4220. [PubMed] [Google Scholar]


