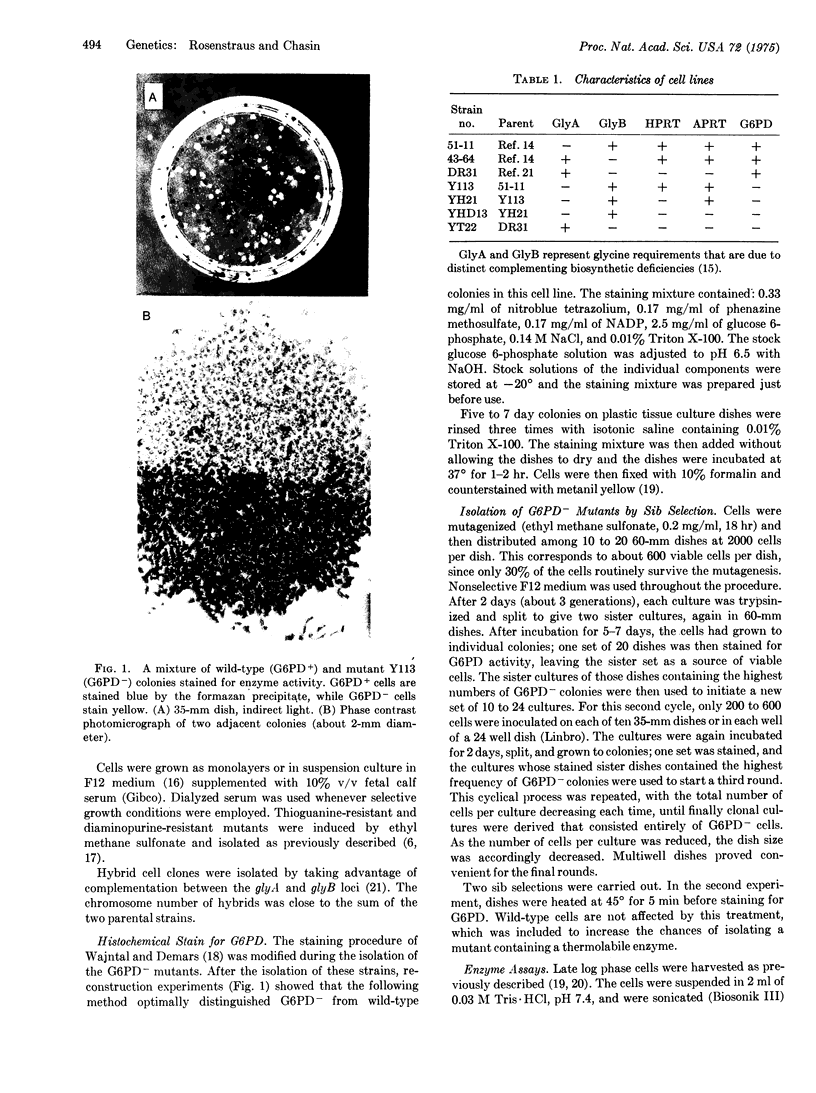
Abstract
Mutants of Chinese hamster ovary cells deficient in glucose-6-phosphate dehydrogenase (D-glucose-6-phosphate: NADP 1-oxidoreducatse, EC 1.1.1.49) activity were isolated after mutagenesis with ethyl methane sulfonate. The mutants were induced at frequencies of about 10-4 and do not differ in growth properties from wild-type cells. They were isolated by means of a sib selection technique coupled with a histochemical stain of colonies for enzyme activity. The lack of enzyme activity is not due to a dissociable inhibitor, and is recessive in hybrid cells. Multiple mutants that lack hypoxanthine phosphoribosyltransferase activity (IMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.8) and adenine phosphoribosyltransferase activity (AMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.7) were isolated by further mutagenesis. By following segregation of wild-type phenotypes from heterozygous multiply marked hybrid cells, it was shown that the genes responsible for glucose-6-phosphate dehydrogenase activity and hypoxanthine phosphoribosyltransferase activity are linked in Chinese hamster cells, in agreement with the location of both on the X chromosome in humans. No linkage to adenosine phosphoribosyltransferase was found. The isolation of mutant cells carrying linked markers should prove useful for studying chromosomal events such as segregation, breakage, recombination, and X-chromosome reactivation.
Full text
PDF
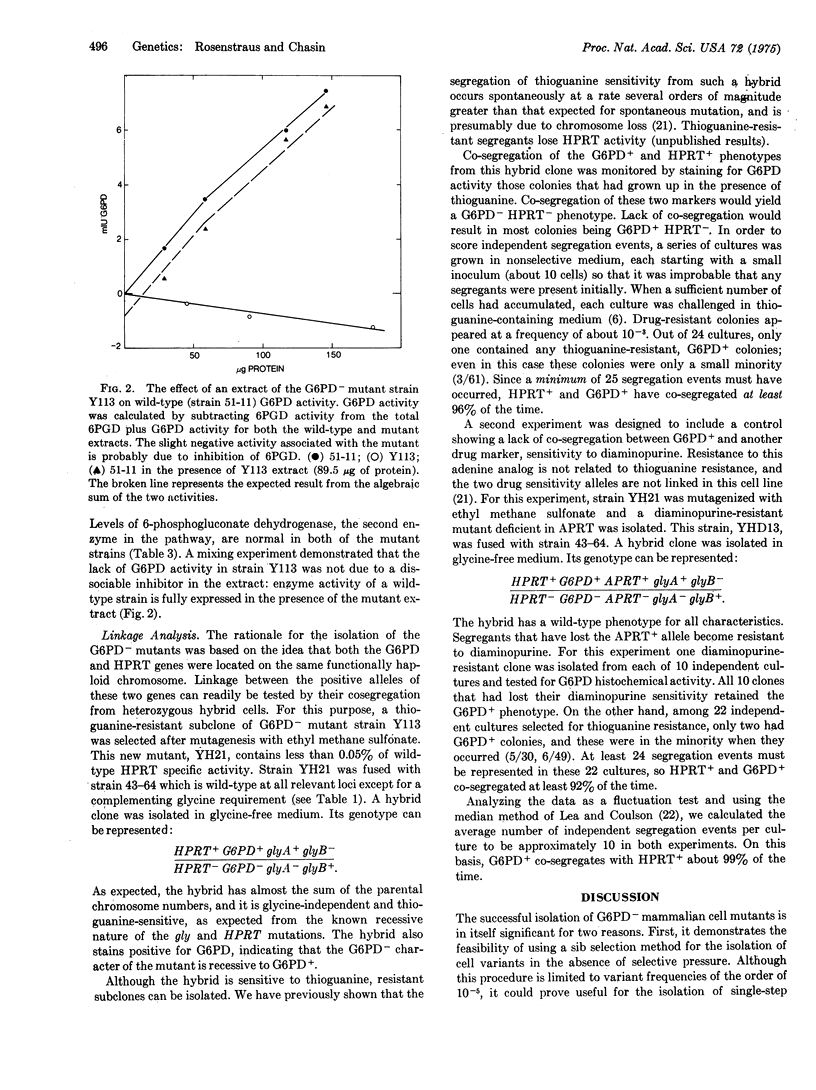

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Roufa D. J., Caskey C. T. Mutations affecting the structure of hypoxanthine: guanine phosphoribosyltransferase in cultured Chinese hamster cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):320–324. doi: 10.1073/pnas.70.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A., Feldman A., Konstam M., Urlaub G. Reversion of a Chinese hamster cell auxotrophic mutant. Proc Natl Acad Sci U S A. 1974 Mar;71(3):718–722. doi: 10.1073/pnas.71.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A. Mutations affecting adenine phosphoribosyl transferase activity in Chinese hamster cells. Cell. 1974 May;2(1):37–41. doi: 10.1016/0092-8674(74)90006-3. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. Non-linkage of induced mutations in Chinese hamster cells. Nat New Biol. 1972 Nov 8;240(97):50–52. doi: 10.1038/newbio240050a0. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. The effect of ploidy on chemical mutagenesis in cultured Chinese hamster cells. J Cell Physiol. 1973 Oct;82(2):299–307. doi: 10.1002/jcp.1040820218. [DOI] [PubMed] [Google Scholar]
- De Luca C., Gioeli R. P. Transhydrogenase activity in mammalian cells in vitro: its possible physiological significance. In Vitro. 1971 Jul-Aug;7(1):13–16. doi: 10.1007/BF02618999. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- GARTLER S. M., GANDINI E., CEPPELLINI R. Glucose-6-phosphate dehydrogenase deficient mutant in human cell culture. Nature. 1962 Feb 10;193:602–603. doi: 10.1038/193602a0. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham R. G., Sattler G. L. Clonal growth of differentiated rabbit cartilage cells. J Cell Physiol. 1968 Oct;72(2):109–114. doi: 10.1002/jcp.1040720205. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F., Chasin L., Puck T. T. Genetics of somatic mammalian cells. X. Complementation analysis of glycine-requiring mutants. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1284–1291. doi: 10.1073/pnas.64.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S. Lest I forget thee, glutathione. Nature. 1969 Oct 11;224(5215):117–120. doi: 10.1038/224117a0. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Marin G. Selection of chromosomal segregants in a "hybrid" line of Syrian hamster fibroblasts. Exp Cell Res. 1969 Sep;57(1):29–36. doi: 10.1016/0014-4827(69)90363-2. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Chase G. A. Human genetics. Annu Rev Genet. 1973;7:435–472. doi: 10.1146/annurev.ge.07.120173.002251. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Beutler E. Useful agents for the study of glutathione metabolism in erythroyctes. Organic hydroperoxides. Biochem J. 1974 May;139(2):289–295. doi: 10.1042/bj1390289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajntal A., DeMars R. A tetrazolium method for distinguishing between cultured human fibroblasts having eiter normal or deficient levels of glucose-6-phosphate dehydrogenase. Biochem Genet. 1967 Jun;1(1):61–64. doi: 10.1007/BF00487737. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Visser R. P., Freeke M. A., Bootsma D. Evidence for linkage of 3-phosphoglycerate kinase, hypoxanthine-guanine-phosphoribosyl transferase, and glucose 6-phosphate dehydrogenase loci in Chinese hamster cells studied by using a relationship between gene multiplicity and enzyme activity. Biochem Genet. 1972 Aug;7(1):33–40. doi: 10.1007/BF00487007. [DOI] [PubMed] [Google Scholar]