Abstract
AIM: To study the formation of intracellular glyceraldehyde-derived advanced glycation end products (Glycer-AGEs) in the presence of high concentrations of fructose.
METHODS: Cells of the human hepatocyte cell line Hep3B were incubated with or without fructose for five days, and the corresponding cell lysates were separated by two-dimensional gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Glycer-AGEs were detected with the anti-Glycer-AGEs antibody. Furthermore, the identification of the proteins that are modified by glyceraldehyde in the presence of high concentrations of fructose was conducted using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The protein and mRNA levels were determined by Western blotting and real-time reverse transcription PCR, respectively.
RESULTS: The results of the two-dimensional gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated a greater amount of Glycer-AGEs in the sample exposed to high concentrations of fructose than in the control. The detected Glycer-AGEs showed isoelectric points in the range of 8.0-9.0 and molecular weights in the range of 60-80 kDa. The heterogeneous nuclear ribonucleoprotein M (hnRNPM), which plays an important role in regulating gene expression by processing heterogeneous nuclear RNAs to form mature mRNAs, was identified as a modified protein using MALDI-TOF-MS. Increasing the concentration of fructose in the medium induced a concentration-dependent increase in the generated Glycer-AGEs. Furthermore, in an experiment using glyceraldehyde, which is a precursor of Glycer-AGEs, hnRNPM was found to be more easily glycated than the other proteins.
CONCLUSION: The results suggest that glyceraldehyde-modified hnRNPM alters gene expression. This change may cause adverse effects in hepatocytes and may serve as a target for therapeutic intervention.
Keywords: Advanced glycation end-products, Fructose, Glycation, Glyceraldehyde, Heterogeneous nuclear ribonucleoprotein M, Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis
Core tip: Excessive intake of fructose contributes to the development of nonalcoholic fatty liver disease and to the progression of the disease to nonalcoholic steatohepatitis. Fructose is metabolized to glyceraldehyde, which is a precursor of glyceraldehyde-derived advanced glycation end-products (Glycer-AGEs). We showed that intracellular Glycer-AGEs were formed in the presence of high concentrations of fructose. Additionally, heterogeneous nuclear ribonucleoprotein M (hnRNPM) was identified as one of the target proteins for glycation. These results suggest that the glyceraldehyde-modified hnRNPM resulting from the exposure of the cells to high concentrations of fructose, alters gene expression and causes adverse effects in hepatocytes.
INTRODUCTION
Fructose is a major component in high fructose corn syrup (HFCS) and sucrose, which are commonly used as sweeteners in beverages and processed foods. The consumption of fructose has increased over the past 40 years, and HFCS consumption has increased rapidly to replace 50% of the sucrose consumption. HFCS is cheaper than sucrose and can be transported easily; moreover, it is more effective in stabilizing the texture of some processed foods than sucrose[1,2]. Increased fructose intake causes many adverse effects such as obesity, dyslipidemia, and insulin resistance, and contributes to the development and progression of nonalcoholic fatty liver disease (NAFLD). NAFLDs ranging from simple steatosis to steatohepatitis include the most common liver diseases worldwide[1-5].
Advanced glycation end-products (AGEs) are formed by the Maillard reaction, a nonenzymatic reaction between the ketones or aldehydes of sugars and the amino groups of proteins. AGEs are associated with aging and diabetes-related pathologic complications[6,7]. This reaction begins with the conversion of reversible Schiff base adducts to more stable covalently bound Amadori rearrangement products. Over the course of days to weeks, these Amadori products undergo further rearrangement reactions to form irreversibly bound moieties known as AGEs[8].
Recent studies have suggested that AGEs can be formed not only from sugars, but also from carbonyl compounds produced as a result of the autoxidation of sugars and from other metabolic pathways[9,10]. Evidence suggests that the glyceraldehyde-derived AGEs (Glycer-AGEs) are associated with diabetes-related pathologic complications[11-13]. Absorbed fructose is selectively metabolized in the liver and later metabolized into glyceraldehyde by aldolase[1,2]. The immunohistochemical analysis of Glycer-AGEs show intense staining in the livers of patients with nonalcoholic steatohepatitis (NASH), which is a form of NAFLD[14]. It is well known that AGE modification adversely alters protein function[15,16]. However, the effects of fructose on the formation of intracellular Glycer-AGEs remain poorly understood.
In this study, we have examined the formation of intracellular Glycer-AGEs in the human hepatocyte cell line Hep3B when exposed to high concentrations of fructose.
MATERIALS AND METHODS
Chemicals
All chemicals were commercial samples of high purity and used as supplied. Glyceraldehyde was purchased from Nakalai Tesque (Kyoto, Japan).
Cell cultures
Hep3B cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, United States) supplemented with 10% fetal bovine serum (Equitech-Bio, Kerrville, TX, United States) under standard cell culture conditions (humidified atmosphere, 5% CO2, 37 °C). Cells (2 × 105 cells/mL) were seeded in various plates or culture dishes (BD Biosciences, Franklin Lakes, NJ, United States) and incubated for 24 h before the start of all experiments. To form intracellular Glycer-AGEs, cells were incubated with or without 0.5-10.0 mmol/L fructose for 120 h.
Preparation of cell lysate
In the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample, cells were washed with ice-cold Ca2+- and Mg2+-free PBS [PBS(-)] and subjected to lysis buffer [25 mmol/L Tris-HCl (pH 7.6), 150 mmol/L sodium chloride, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, and 1× protease inhibitor cocktail (Thermo Fisher Scientific Inc., Waltham, MA, United States)]. Subsequently, cell lysates were passed through a syringe several times for further homogenization, and they were centrifuged at 12000× g for 10 min at 4 °C. After the protein concentrations were measured, cell lysates were dissolved in LDS sample buffer (Invitrogen of Thermo Fisher Scientific) containing 10% sample reducing agent (Invitrogen) and boiled for 10 min at 70 °C.
In the two-dimensional gradient SDS-PAGE sample, cells were washed with ice-cold PBS(-) and subjected to 10% trichloroacetic acid for 30 min at 4 °C. Subsequently, collected cells were washed with PBS(-) and dissolved in urea buffer (2 mol/L thiourea, 7 mol/L urea, 3% CHAPS, 1% Triton X-100, 10% sample reducing agent) for 30 min at 4 °C. The resulting suspension was centrifuged at 20000× g for 30 min at 4 °C.
Protein concentrations were measured using the Bradford assay (Bio-Rad Laboratories Inc., Hercules, CA, United States).
Two-dimensional gradient SDS-PAGE and mass spectrometry protein identification
After measuring the protein concentrations, cell lysates that modified acrylamide (100 μg) were separated in agar gel (pH range: 3-10 or 5-10) (ATTO, Tokyo, Japan) and 5-20% SDS-polyacrylamide gradient gel (ATTO). All processes were performed according to the manufacturer’s instructions.
After two-dimensional gradient SDS-PAGE, the gel was stained with EzStain Aqua (ATTO) for 3 h and washed with distilled water. The selected spots were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis. We consigned MALDI-TOF-MS analysis to Genomine, Inc (Kyungbuk, Korea).
Western blot analysis
Cell lysates were separated by electrophoresis and then electro-transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Billerica, MA, United States). Membranes were blocked for 60 min by using the PVDF blocking reagent for Can Get Signal (Toyobo, Osaka, Japan). After washing with PBS containing 0.05% Tween 20 (PBS-T), the membranes were incubated with rabbit anti-Glycer-AGEs antibody, mouse anti-heterogeneous nuclear ribonucleoprotein M (hnRNPM) antibody (Millipore), mouse anti-β-actin antibody (Santa Cruz Biotechnology, Dallas, TX, United States), or rabbit anti-GAPDH antibody (GeneTex, Irvine, CA, United States) in Can Get Signal Solution 1 (Toyobo) for 1 h. Subsequently, the membranes were washed three times with PBS-T and incubated with anti-rabbit IgG antibody (GeneTex) or anti-mouse IgG antibody (Dako of Agilent Technologies, Santa Clara, CA, United States) in Can Get Signal Solution 2 (Toyobo) for 1 h. After five additional washes with PBS-T, immunoreactive proteins were detected using ECL Prime Western Blotting Detection Reagents and Amersham hyperfilm ECL (GE Healthcare Ltd., Little Chalfont, Buckinghamshire, United Kingdom).
Neutralization of rabbit anti-Glycer-AGEs antibody
Glycer-AGEs were prepared as described previously[17]. Briefly, 25 mg/mL of bovine serum albumin (BSA; A0281, Sigma-Aldrich) was incubated at 37 °C for 7 d under sterile conditions with 0.1 mol/L glyceraldehyde and 5 mmol/L diethylenetriaminepentaacetic acid (Dojindo Laboratories, Kumamoto, Japan) in 0.2 mol/L phosphate buffer (pH 7.4). As a control, unglycated BSA was incubated under the same conditions, but without glyceraldehyde. The unglycated and glycated albumin were purified using a PD-10 column (GE Healthcare Ltd.) and dialysis against PBS. All preparations were tested for endotoxin using the Endospecy ES-20S system (Seikagaku Co., Tokyo, Japan). Protein concentrations were determined using the Dc protein assay reagent (Bio-Rad Laboratories) using BSA as a standard.
The amount of Glycer-AGEs-BSA required to neutralize rabbit anti-Glycer-AGE antibody was calculated based on a standard curve of Glycer-AGEs-BSA in a competitive ELISA. The antibody was incubated with 50 μg/mL of Glycer-AGEs-BSA for 1 h at room temperature and it was used for the subsequent experiments.
Real-time reverse transcription-PCR
Total RNA was isolated from Hep3B cells using ISOGEN II (Nippon Gene, Tokyo, Japan), and cDNA was synthesized using random primers and reverse transcriptase. Real-time reverse transcription-PCR was performed using a Smart Cycler II System (Takara, Shiga, Japan), as previously described[18]. The primers used were as follows: hnRNPM, 5′-GAG GCC ATG CTC CTG GG-3′ and 5′-TTT AGC ATC TTC CAT GTG AAA TCG-3′; and β-actin, 5′-TCC ACC TCC AGC AGA TGT GG-3′ and 5′-GCA TTT GCG GTG GAC GAT-3′.
Statistical analysis
All experiments were performed in duplicate and repeated at least two or three times. Each experiment yielded essentially identical results. Data are expressed as the mean ± SD. The significance of differences between group means was determined using a t-test. P < 0.05 was defined as significant.
RESULTS
Exposure to high fructose concentrations enhanced the formation of intracellular Glycer-AGEs in Hep3B cells
We examined whether intracellular Glycer-AGEs were formed by exposure to high fructose concentrations. In a Western blot analysis using the anti-Glycer-AGEs antibody after two-dimensional gel electrophoresis, various spots were detected in the control and fructose samples (Figure 1A and B). Western blot analysis using neutralized anti-Glycer-AGEs antibody was used to clarify the nonspecific spots, which showed no difference in their expression levels or expression pattern between the two samples (Figure 1C and D). In the sample that was exposed to high fructose concentrations, the Glycer-AGE spots were observed to have isoelectric points in the range of 8.0-9.0 and molecular weights in the range of 60-80 kDa (Figure 1B arrow).
Figure 1.
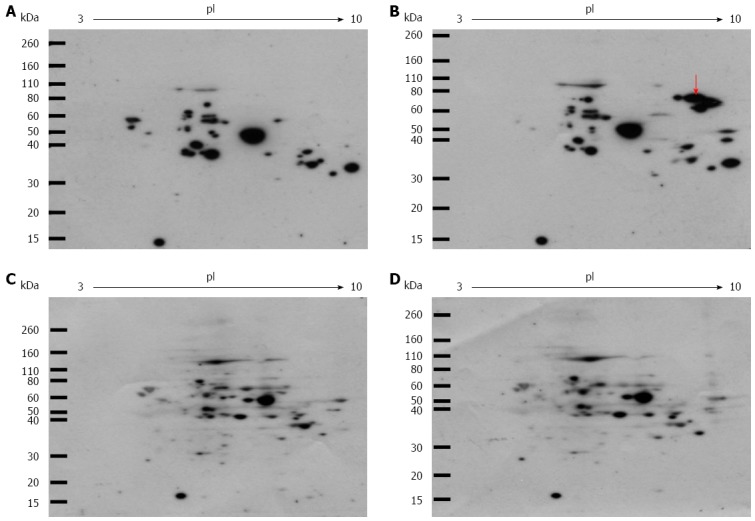
Western blot analysis of intracellular glyceraldehyde-derived advanced glycation end-products. Cells were incubated with or without 2 mmol/L fructose for 5 d. Cell lysates (100 μg of protein/gel) were separated on two-dimensional gradient sodium dodecyl sulfate-polyacrylamide electrophoresis and probed with Anti-glyceraldehyde-derived advanced glycation end-products (Glycer-AGEs) antibody (A, B), or anti-Glycer-AGEs antibody neutralized by an excess of Glycer-AGEs-bovine serum albumin (C, D); A,C: control samples; B,D: Fructose samples. The arrow shows spots where a difference was seen.
Identification of the proteins modified by glyceraldehyde upon exposure to high fructose concentrations
After two-dimensional gel electrophoresis, for the sample exposed to high concentrations of fructose, the gel was stained with Coomassie blue (Figure 2). The gel spots that matched those detected with anti-Glycer-AGEs antibody were identified by mass spectrometry analysis. We identified four spots of Glycer-AGEs (Figure 2 circle), and interestingly, all four proteins were hnRNPM (Table 1). The spots detected with anti-Glycer-AGEs antibody were in accordance with the spots detected with anti-hnRNPM antibody (Figure 3).
Figure 2.
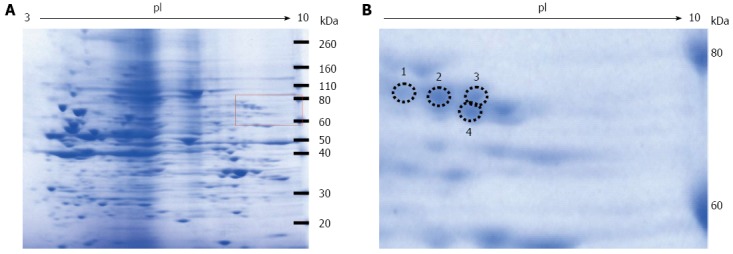
Coomassie blue staining of the two-dimensional gradient gel. A: High fructose exposure samples were separated on two-dimensional gradient sodium dodecyl sulfate-polyacrylamide electrophoresis and then stained with EzStain Aqua; B: Higher magnification of the square region of the left panel. The number and the position of the four selected spots are indicated by circles.
Table 1.
Identification of intracellular glyceraldehyde-derived advanced glycation end-products formed by high fructose exposure
| Spot No. | Protein name | Score | Mass values matched | Coverage | Calculated isoelectric point | Nominal mass (kDa) |
| 1 | Heterogeneous nuclear ribonucleoprotein M | 90 | 13 | 20% | 8.8 | 78 |
| 2 | Heterogeneous nuclear ribonucleoprotein M | 99 | 18 | 27% | 8.8 | 78 |
| 3 | Heterogeneous nuclear ribonucleoprotein M | 84 | 16 | 28% | 8.8 | 78 |
| 4 | Heterogeneous nuclear ribonucleoprotein M | 76 | 14 | 22% | 8.8 | 78 |
Selected spots were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. The number and the position of the four selected spots refer to the numbered spots in Figure 2B.
Figure 3.
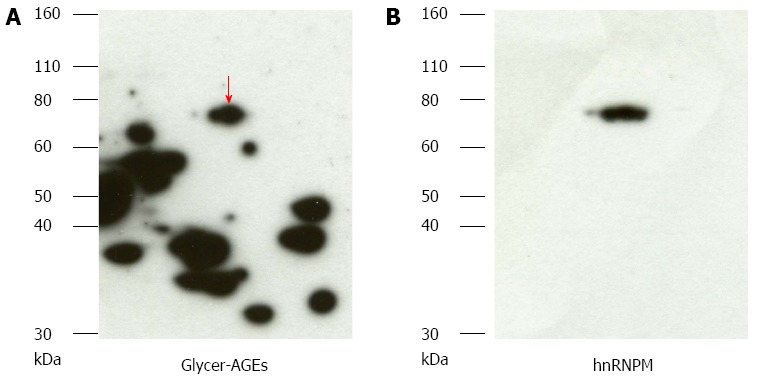
Detection of glyceraldehyde modification of heterogeneous nuclear ribonucleoprotein M by high fructose exposure. A: Samples exposed to high fructose concentrations were separated on two-dimensional gradient sodium dodecyl sulfate-polyacrylamide electrophoresis and probed with anti-glyceraldehyde-derived advanced glycation end-products (Glycer-AGEs) antibody. The arrow shows spots where a difference was seen; B: They were reprobed with heterogeneous nuclear ribonucleoprotein M (hnRNPM) antibody.
hnRNPM is the target protein of glycation
We examined the effects of exposure to high fructose concentrations on the modification of hnRNPM by glyceraldehyde. Fructose exposure induced a concentration-dependent increase in glyceraldehyde modification of hnRNPM, but it did not increase the protein or mRNA levels of hnRNPM (Figure 4).
Figure 4.
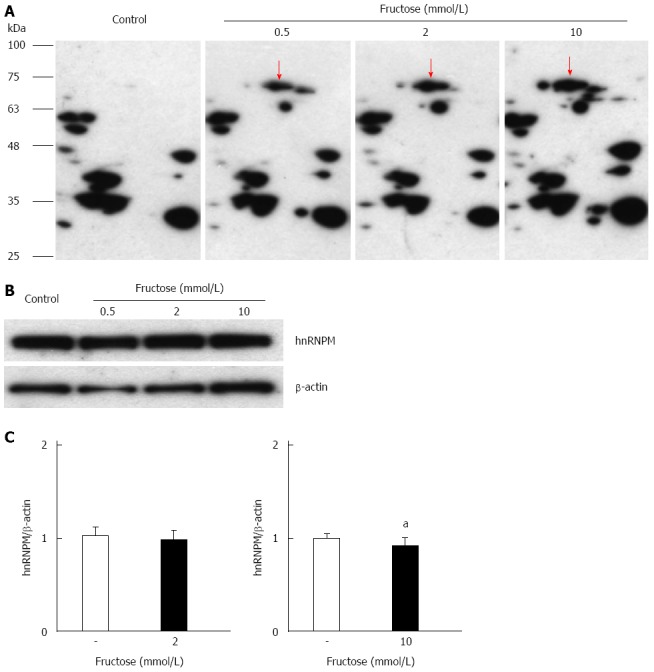
Effects of glyceraldehyde modification of heterogeneous nuclear ribonucleoprotein M by high fructose exposure. Cells were incubated with 0.5-10.0 mmol/L fructose for 5 d. A: Cell lysates were separated on two-dimensional gradient sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and the glyceraldehyde modification of heterogeneous nuclear ribonucleoprotein M (hnRNPM) was determined by probing with anti-Glycer-AGEs antibody. The arrow shows spots identified with hnRNPM; B: Cell lysates were separated by SDS-PAGE and probed with anti-hnRNPM antibody. Equal protein loading was determined using anti-β-actin antibody; C: The levels of mRNA expression were analyzed by real-time RT-PCR, and the result was normalized to β-actin. Data are shown as mean ± SD (n = 6); aP < 0.05 vs control. Left panel: With 2 mmol/L fructose, right panel: With 10 mmol/L fructose.
Next, we examined the effects of the modification of hnRNPM by glyceraldehyde, which is a precursor of Glycer-AGEs. After 5 d in culture, the cells were incubated with or without 4 mmol/L glyceraldehyde for 6 h. Western blot analysis using anti-Glycer-AGEs antibody after two-dimensional gel electrophoresis showed that the various spots of the glyceraldehyde sample were detected more strongly than the control sample (Figure 5A and B). The spot of hnRNPM was also included (Figure 5B arrow). Furthermore, Western blot analysis using anti-hnRNPM antibody showed the bands that shifted to the top, whereas the hnRNPM protein of 68 kDa was decreased by the addition of glyceraldehyde. On the other hand, such a change was not seen in β-actin or GAPDH (Figure 5C).
Figure 5.
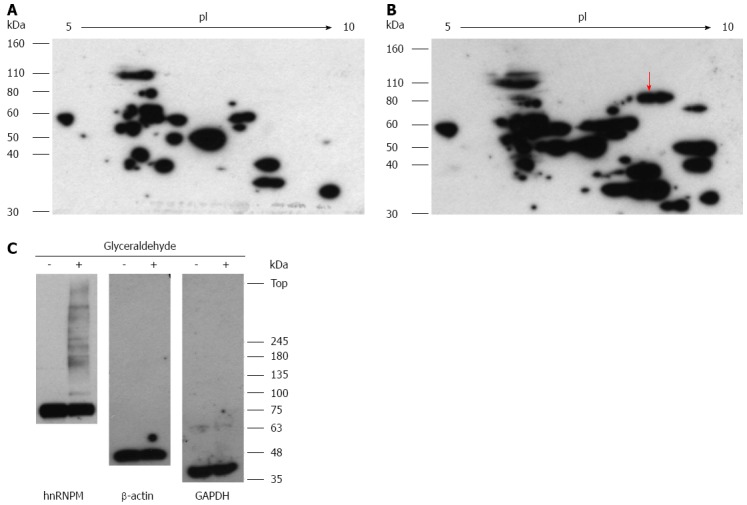
Effects of glyceraldehyde modification of heterogeneous nuclear ribonucleoprotein M. Hep3B cells were incubated for 5 d and incubated with or without 4 mmol/L glyceraldehyde for 6 h. Cell lysates were separated on two-dimensional gradient sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) (A, B); or SDS-PAGE and probed with anti-Glycer-AGEs antibody (C). A: Control sample; B: Glyceraldehyde sample. The arrow shows spot identified with heterogeneous nuclear ribonucleoprotein M (hnRNPM).
DISCUSSION
Glycer-AGEs cause various intracellular and extracellular adverse effects. Extracellular Glycer-AGEs interact with receptors for AGEs and increase oxidative stress by the production of reactive oxygen species in the cell[19]. On the other hand, intracellular Glycer-AGEs cause a functional change in the protein itself[11,12]. We previously observed that glyceraldehyde, which is a precursor of Glycer-AGEs, increases the intracellular Glycer-AGEs in Hep3B cells. Among the intracellular Glycer-AGEs that were formed, heat shock cognate 70 was identified as a glyceraldehyde-modified protein, and the modification by glyceraldehyde reduced the activity of the protein[18].
In the liver, glyceraldehyde is believed to be produced by two pathways: the glycolytic pathway and the fructose metabolic pathway. Fructose metabolism involves fructokinase and is especially important in the liver after food intake. Fructose is phosphorylated to fructose-1-phosphate (F-1-P) by a specific kinase, and the liver aldolase B can cleave F-1-P to produce dihydroxyacetone phosphate and glyceraldehyde[20]. Therefore, the liver more readily accumulates glyceraldehyde than other organs. In this study, we have described the formation of intracellular Glycer-AGEs induced by high fructose exposure in Hep3B cells.
Whether the in vitro addition of fructose to hepatocytes is physiologically relevant is an important issue for further research. Le et al[21] demonstrated that the baseline fructose level in the human peripheral vein was about 5 μmol/L, and after ingestion of 24 oz of HFCS-sweetened beverages, this level increased to 0.3 mmol/L. Fructose absorbed in the small intestine is carried to the portal vein. Therefore, it is expected that the portal vein has higher fructose levels than the peripheral vein. Furthermore, Sugimoto et al[22] demonstrated that the peak concentration of portal fructose was approximately ten times that of peripheral fructose after sucrose ingestion in rats. This is consistent with our findings, which show that Glycer-AGEs were detected even when the concentration of fructose was less than 3 mmol/L.
Heterogeneous nuclear ribonucleoproteins, which directly bind to nascent RNA polymerase II transcripts, play an important role in processing heterogeneous nuclear RNAs to form mature mRNAs and in regulating gene expression. It is known that hnRNPM appears as a cluster of four proteins, M1-4, of 64-68 kDa in two-dimensional gel electrophoresis[23]. We showed that a Glycer-AGE, which we identified to be hnRNPM, is a modified protein that was detected in cells exposed to high fructose concentrations, and this exposure induced a concentration-dependent increase in Glycer-AGEs (glyceraldehyde-modified hnRNPM). It is known that the ketones or aldehydes of sugars can modify the side chains of lysine and arginine residues in proteins[24,25]; Cotham et al[26] demonstrated that dicarbonyl compounds react primarily with arginine residues. Lysine and arginine, amino acids that have a basic side chain, are important in RNA-protein interactions[27-31]. In particular, the function of hnRNPM may be to influence lysine sumoylation and arginine methylation, which are post-translational modifications[32,33]. Furthermore, hnRNPM possesses an unusual hexapeptide-repeat region rich in methionine and arginine residues (MR repeat motif)[23], and this motif may participate in the formation of Glycer-AGEs. In experiments where glyceraldehyde is externally added, the bands that shifted to the top appeared, while the level of 68-kDa hnRNPM protein was decreased. It is well known that the glycation of proteins forms intermolecular crosslinking[34-36]. Therefore, it is thought that this phenomenon of crosslinking characterizes glycation. However, no crosslinking was observed in β-actin or GAPDH. This suggests that the hnRNPM protein was more easily glycated than the other proteins.
Finally, in order to understand hnRNPM function, we used RIP-Chip analysis in Hep3B cells to identify the genes that are controlled by hnRNPM. The results showed that apolipoprotein E (APOE) and fibrinogen-like 1 (FGL1) were included in the top 50 such genes (Table 2). It is known that APOE is a ligand for low-density lipoprotein receptors and participates in the transport of cholesterol and other lipids[37]. It was reported that the APOE polymorphism is significantly associated with NASH[38]. Furthermore, APOE-deficient, APOE*3-Leiden (variant form of APOE), and APOE2 knock-in (APOE2ki) mice are widely used as models of liver steatosis[39-43]. These mice were maintained on a high-fat diet to allow the development of NASH[40-43]. It is also known that FGL1 expression is induced after liver injury; FGL1 stimulates hepatocyte proliferation and protects hepatocytes from injury[44-46].
Table 2.
Top 50 RNA targets of heterogeneous nuclear ribonucleoprotein M in Hep3B cells
| Rank | Gene title | Gene symbol |
| 1 | Apolipoprotein E | APOE |
| 2 | Albumin | ALB |
| 3 | RNA, 28S ribosomal 1 | RN28S1 |
| 4 | PQ loop repeat containing 2 | PQLC2 |
| 5 | Zinc finger protein 865 | ZNF865 |
| 6 | Unknown | A_33_P3396434 |
| 7 | lincRNA | XLOC_l2_004940 |
| 8 | CD63 molecule | CD63 |
| 9 | Eukaryotic translation elongation factor 1 alpha 1 | EEF1A1 |
| 10 | G protein-coupled receptor 155 | GPR155 |
| 11 | Family with sequence similarity 74, member A4 | FAM74A4 |
| 12 | LOC284600 | ENST00000448179 |
| 13 | LOC100506453 | ENST00000381105 |
| 14 | ATP synthase, H+ transporting, mitochondrial Fo complex, subunit G | ATP5L |
| 15 | Unknown | A_33_P3370515 |
| 16 | Proteasome (prosome, macropain) 26S subunit, non-ATPase, 2 | PSMD2 |
| 17 | Ribosomal protein L23 | RPL23 |
| 18 | Proteasome (prosome, macropain) subunit, beta type, 1 | PSMB1 |
| 19 | Arsenic (+3 oxidation state) methyltransferase | AS3MT |
| 20 | Heat shock 60 kDa protein 1 (chaperonin) | HSPD1 |
| 21 | H2A histone family, member V | H2AFV |
| 22 | lincRNA | XLOC_l2_015885 |
| 23 | Fucosyltransferase 6 (alpha (1,3) fucosyltransferase) | FUT6 |
| 24 | Cytochrome P450, family 2, subfamily W, polypeptide 1 | CYP2W1 |
| 25 | Unknown | A_33_P3275826 |
| 26 | Microtubule-associated protein 4 | MAP4 |
| 27 | lincRNA | XLOC_l2_002910 |
| 28 | X-ray repair complementing defective repair in Chinese hamster cells 6 | XRCC6 |
| 29 | Solute carrier family 16, member 12 (monocarboxylic acid transporter 12) | SLC16A12 |
| 30 | Actin, beta | ACTB |
| 31 | Unknown | A_33_P3315763 |
| 32 | Mitochondrial carrier 1 | MTCH1 |
| 33 | Stromal cell-derived factor 2-like 1 | SDF2L1 |
| 34 | Chromosome 19 open reading frame 10 | C19orf10 |
| 35 | Kinesin family member 1C | KIF1C |
| 36 | Fibrinogen-like 1 | FGL1 |
| 37 | RAN, member RAS oncogene family | RAN |
| 38 | Unknown | ENST00000400768 |
| 39 | Homer homolog 3 (Drosophila) | HOMER3 |
| 40 | HscB iron-sulfur cluster co-chaperone homolog (E. coli) | HSCB |
| 41 | Canopy 2 homolog (zebrafish) | CNPY2 |
| 42 | Thymidine phosphorylase | TYMP |
| 43 | F11 receptor | F11R |
| 44 | Mastermind-like 1 (Drosophila) | MAML1 |
| 45 | Electron-transfer-flavoprotein, beta polypeptide | ETFB |
| 46 | Heterogeneous nuclear ribonucleoprotein A1 | HNRNPA1 |
| 47 | Alpha-1-microglobulin/bikunin precursor | AMBP |
| 48 | Calreticulin | CALR |
| 49 | Solute carrier family 25 (mitochondrial carrier; phosphate carrier), member 3 | SLC25A3 |
| 50 | Alpha-2-HS-glycoprotein | AHSG |
RNA-hnRNPM complexes were co-immunoprecipitated with anti-heterogeneous nuclear ribonucleoprotein M antibody bound to protein G-magnetic beads, and RNAs were isolated and identified by microarray analysis.
In conclusion, among the intracellular Glycer-AGEs that were formed, hnRNPM was identified as a glyceraldehyde-modified protein, and it was more easily glycated than the other proteins. Therefore, we believe that the gene responsible for NAFLD and NASH is among those whose expression is regulated by hnRNPM. These results suggest that intracellular Glycer-AGEs may play a critical role in the pathogenesis of NAFLD and NASH and may serve as potential targets for therapeutic intervention. A further analysis of the target genes of hnRNPM will be necessary in the future.
COMMENTS
Background
Excessive intake of fructose contributes to the development of nonalcoholic fatty liver disease and to the progression of the disease to nonalcoholic steatohepatitis (NASH). Fructose is metabolized to glyceraldehyde, which is a precursor of glyceraldehyde-derived (Glycer)-advanced glycation end-products (AGEs). AGEs are formed by the Maillard reaction, a nonenzymatic reaction that occurs between the ketones or aldehydes of sugars and the amino groups of proteins. AGEs are associated with aging and diabetes-related pathologic complications. The immunohistochemical analysis of Glycer-AGEs shows an intense staining in the livers of patients with NASH. However, the effects of fructose on the formation of intracellular Glycer-AGEs remain poorly understood.
Research frontiers
Evidence suggests that among the various AGEs, Glycer-AGEs are associated with diabetes-related pathologic complications, NASH, and cancer. The extracellular Glycer-AGEs-receptor for AGE signaling pathway is well understood, and it has been previously shown that AGE modifications adversely alter protein functions. In this study, the authors examined the formation of intracellular Glycer-AGEs in the presence of high concentrations of fructose.
Innovations and breakthroughs
This study reported the formation of intracellular Glycer-AGEs and identified the glyceraldehyde-modified proteins by exposing the cells to high fructose concentrations.
Applications
The experimental data can be used in further studies for therapeutic intervention using these Glycer-AGEs as potential targets.
Peer-review
The study by Takino and coworkers focuses on the molecular mechanism with which excessive intake of fructose contributes to the development of an emerging and worrisome pathology such as nonalcoholic fatty liver disease and its inflammatory progression, NASH.
Footnotes
Supported by Grants from the Japan Society for the Promotion of Science, No. 22300264 and No. 25282029.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 20, 2014
First decision: August 15, 2014
Article in press: October 15, 2014
P- Reviewer: Calamita G S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
References
- 1.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 2.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57:2525–2531. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 3.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299:E685–E694. doi: 10.1152/ajpendo.00283.2010. [DOI] [PubMed] [Google Scholar]
- 4.Attar BM, Van Thiel DH. Current concepts and management approaches in nonalcoholic fatty liver disease. Scientific World Journal. 2013;2013:481893. doi: 10.1155/2013/481893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlon BA, Beasley JM, Aebersold K, Jhangiani SS, Wylie-Rosett J. Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD) Nutrients. 2013;5:4093–4114. doi: 10.3390/nu5104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.al-Abed Y, Kapurniotu A, Bucala R. Advanced glycation end products: detection and reversal. Methods Enzymol. 1999;309:152–172. doi: 10.1016/s0076-6879(99)09013-8. [DOI] [PubMed] [Google Scholar]
- 7.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi M, Makita Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr Mol Med. 2001;1:305–315. doi: 10.2174/1566524013363735. [DOI] [PubMed] [Google Scholar]
- 9.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 10.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109–116. [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, Kameda Y, Makita Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000;59:1094–1105. doi: 10.1093/jnen/59.12.1094. [DOI] [PubMed] [Google Scholar]
- 12.Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, Yamamoto H, Takeuchi M, Makita Z. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290:973–978. doi: 10.1006/bbrc.2001.6312. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, Makita Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem. 2002;277:20309–20315. doi: 10.1074/jbc.M202634200. [DOI] [PubMed] [Google Scholar]
- 14.Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, Ochi H, Nonaka M, Nabeshima Y, Inoue M, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112–1119. doi: 10.1111/j.1440-1746.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamelin M, Mary J, Vostry M, Friguet B, Bakala H. Glycation damage targets glutamate dehydrogenase in the rat liver mitochondrial matrix during aging. FEBS J. 2007;274:5949–5961. doi: 10.1111/j.1742-4658.2007.06118.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med. 2000;6:114–125. [PMC free article] [PubMed] [Google Scholar]
- 18.Takino J, Kobayashi Y, Takeuchi M. The formation of intracellular glyceraldehyde-derived advanced glycation end-products and cytotoxicity. J Gastroenterol. 2010;45:646–655. doi: 10.1007/s00535-009-0193-9. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Iwaki M, Shimogaito N, Wu X, Yamagishi S, Takeuchi M. TAGE (toxic AGEs) theory in diabetic complications. Curr Mol Med. 2006;6:351–358. doi: 10.2174/156652406776894536. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi M, Yamagishi S. Alternative routes for the formation of glyceraldehyde-derived AGEs (TAGE) in vivo. Med Hypotheses. 2004;63:453–455. doi: 10.1016/j.mehy.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, Johnson RJ, Johnson JA. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61:641–651. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto K, Hosotani T, Kawasaki T, Nakagawa K, Hayashi S, Nakano Y, Inui H, Yamanouchi T. Eucalyptus leaf extract suppresses the postprandial elevation of portal, cardiac and peripheral fructose concentrations after sucrose ingestion in rats. J Clin Biochem Nutr. 2010;46:205–211. doi: 10.3164/jcbn.09-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datar KV, Dreyfuss G, Swanson MS. The human hnRNP M proteins: identification of a methionine/arginine-rich repeat motif in ribonucleoproteins. Nucleic Acids Res. 1993;21:439–446. doi: 10.1093/nar/21.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed MU, Dunn JA, Walla MD, Thorpe SR, Baynes JW. Oxidative degradation of glucose adducts to protein. Formation of 3-(N epsilon-lysino)-lactic acid from model compounds and glycated proteins. J Biol Chem. 1988;263:8816–8821. [PubMed] [Google Scholar]
- 25.Iijima K, Murata M, Takahara H, Irie S, Fujimoto D. Identification of N(omega)-carboxymethylarginine as a novel acid-labileadvanced glycation end product in collagen. Biochem J. 2000;347 Pt 1:23–27. [PMC free article] [PubMed] [Google Scholar]
- 26.Cotham WE, Metz TO, Ferguson PL, Brock JW, Hinton DJ, Thorpe SR, Baynes JW, Ames JM. Proteomic analysis of arginine adducts on glyoxal-modified ribonuclease. Mol Cell Proteomics. 2004;3:1145–1153. doi: 10.1074/mcp.M400002-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Jones S, Daley DT, Luscombe NM, Berman HM, Thornton JM. Protein-RNA interactions: a structural analysis. Nucleic Acids Res. 2001;29:943–954. doi: 10.1093/nar/29.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Jeong E, Lee SW, Han K. Computational analysis of hydrogen bonds in protein-RNA complexes for interaction patterns. FEBS Lett. 2003;552:231–239. doi: 10.1016/s0014-5793(03)00930-x. [DOI] [PubMed] [Google Scholar]
- 29.Lejeune D, Delsaux N, Charloteaux B, Thomas A, Brasseur R. Protein-nucleic acid recognition: statistical analysis of atomic interactions and influence of DNA structure. Proteins. 2005;61:258–271. doi: 10.1002/prot.20607. [DOI] [PubMed] [Google Scholar]
- 30.Ellis JJ, Broom M, Jones S. Protein-RNA interactions: structural analysis and functional classes. Proteins. 2007;66:903–911. doi: 10.1002/prot.21211. [DOI] [PubMed] [Google Scholar]
- 31.Ciriello G, Gallina C, Guerra C. Analysis of interactions between ribosomal proteins and RNA structural motifs. BMC Bioinformatics. 2010;11 Suppl 1:S41. doi: 10.1186/1471-2105-11-S1-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vertegaal AC, Ogg SC, Jaffray E, Rodriguez MS, Hay RT, Andersen JS, Mann M, Lamond AI. A proteomic study of SUMO-2 target proteins. J Biol Chem. 2004;279:33791–33798. doi: 10.1074/jbc.M404201200. [DOI] [PubMed] [Google Scholar]
- 33.Blackwell E, Ceman S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Mol Reprod Dev. 2012;79:163–175. doi: 10.1002/mrd.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acharya AS, Cho YJ, Manjula BN. Cross-linking of proteins by aldotriose: reaction of the carbonyl function of the keto amines generated in situ with amino groups. Biochemistry. 1988;27:4522–4529. doi: 10.1021/bi00412a045. [DOI] [PubMed] [Google Scholar]
- 35.Raabe HM, Höpner JH, Notbohm H, Sinnecker GH, Kruse K, Müller PK. Biochemical and biophysical alterations of the 7S and NC1 domain of collagen IV from human diabetic kidneys. Diabetologia. 1998;41:1073–1079. doi: 10.1007/s001250051032. [DOI] [PubMed] [Google Scholar]
- 36.Yan H, Harding JJ. Carnosine inhibits modifications and decreased molecular chaperone activity of lens alpha-crystallin induced by ribose and fructose 6-phosphate. Mol Vis. 2006;12:205–214. [PubMed] [Google Scholar]
- 37.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 38.Sazci A, Akpinar G, Aygun C, Ergul E, Senturk O, Hulagu S. Association of apolipoprotein E polymorphisms in patients with non-alcoholic steatohepatitis. Dig Dis Sci. 2008;53:3218–3224. doi: 10.1007/s10620-008-0271-5. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimatsu M, Terasaki Y, Sakashita N, Kiyota E, Sato H, van der Laan LJ, Takeya M. Induction of macrophage scavenger receptor MARCO in nonalcoholic steatohepatitis indicates possible involvement of endotoxin in its pathogenic process. Int J Exp Pathol. 2004;85:335–343. doi: 10.1111/j.0959-9673.2004.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiri-Sverdlov R, Wouters K, van Gorp PJ, Gijbels MJ, Noel B, Buffat L, Staels B, Maeda N, van Bilsen M, Hofker MH. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Lalloyer F, Wouters K, Baron M, Caron S, Vallez E, Vanhoutte J, Baugé E, Shiri-Sverdlov R, Hofker M, Staels B, et al. Peroxisome proliferator-activated receptor-alpha gene level differently affects lipid metabolism and inflammation in apolipoprotein E2 knock-in mice. Arterioscler Thromb Vasc Biol. 2011;31:1573–1579. doi: 10.1161/ATVBAHA.110.220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bieghs V, Van Gorp PJ, Wouters K, Hendrikx T, Gijbels MJ, van Bilsen M, Bakker J, Binder CJ, Lütjohann D, Staels B, et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7:e30668. doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lombardo E, van Roomen CP, van Puijvelde GH, Ottenhoff R, van Eijk M, Aten J, Kuiper J, Overkleeft HS, Groen AK, Verhoeven AJ, et al. Correction of liver steatosis by a hydrophobic iminosugar modulating glycosphingolipids metabolism. PLoS One. 2012;7:e38520. doi: 10.1371/journal.pone.0038520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hara H, Uchida S, Yoshimura H, Aoki M, Toyoda Y, Sakai Y, Morimoto S, Fukamachi H, Shiokawa K, Hanada K. Isolation and characterization of a novel liver-specific gene, hepassocin, upregulated during liver regeneration. Biochim Biophys Acta. 2000;1492:31–44. doi: 10.1016/s0167-4781(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 45.Yan J, Ying H, Gu F, He J, Li YL, Liu HM, Xu YH. Cloning and characterization of a mouse liver-specific gene mfrep-1, up-regulated in liver regeneration. Cell Res. 2002;12:353–361. doi: 10.1038/sj.cr.7290137. [DOI] [PubMed] [Google Scholar]
- 46.Demchev V, Malana G, Vangala D, Stoll J, Desai A, Kang HW, Li Y, Nayeb-Hashemi H, Niepel M, Cohen DE, et al. Targeted deletion of fibrinogen like protein 1 reveals a novel role in energy substrate utilization. PLoS One. 2013;8:e58084. doi: 10.1371/journal.pone.0058084. [DOI] [PMC free article] [PubMed] [Google Scholar]


