Abstract
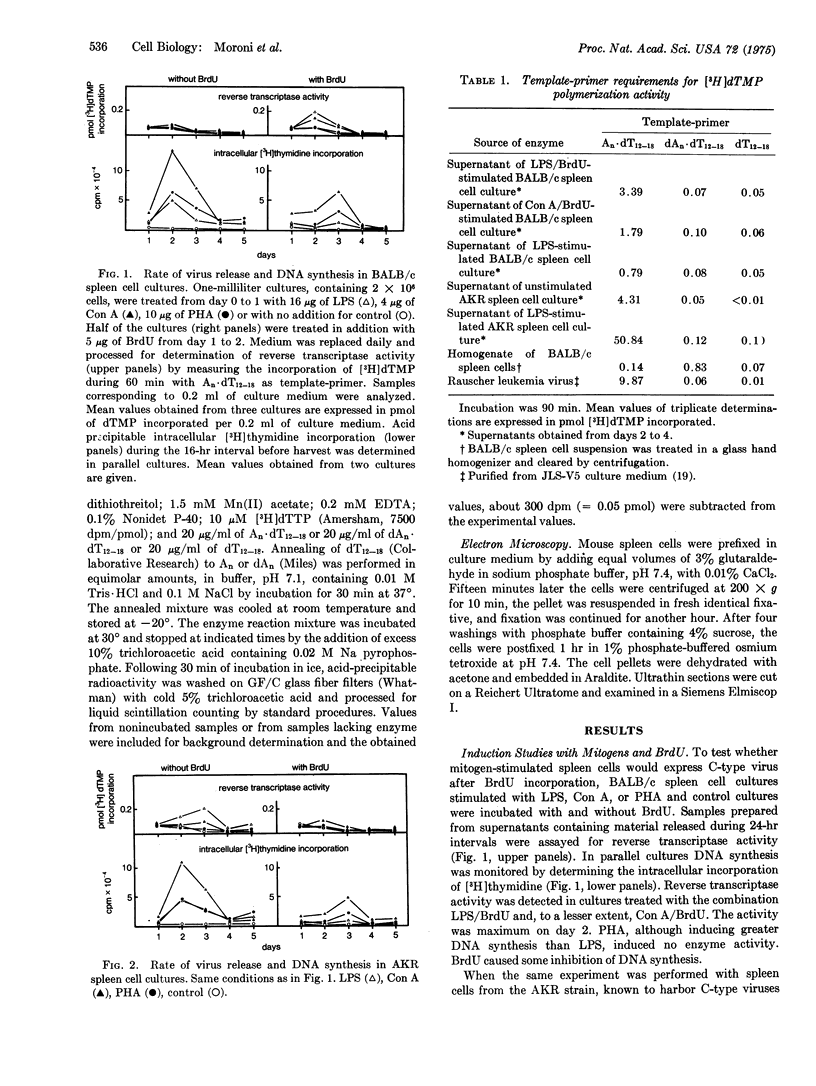
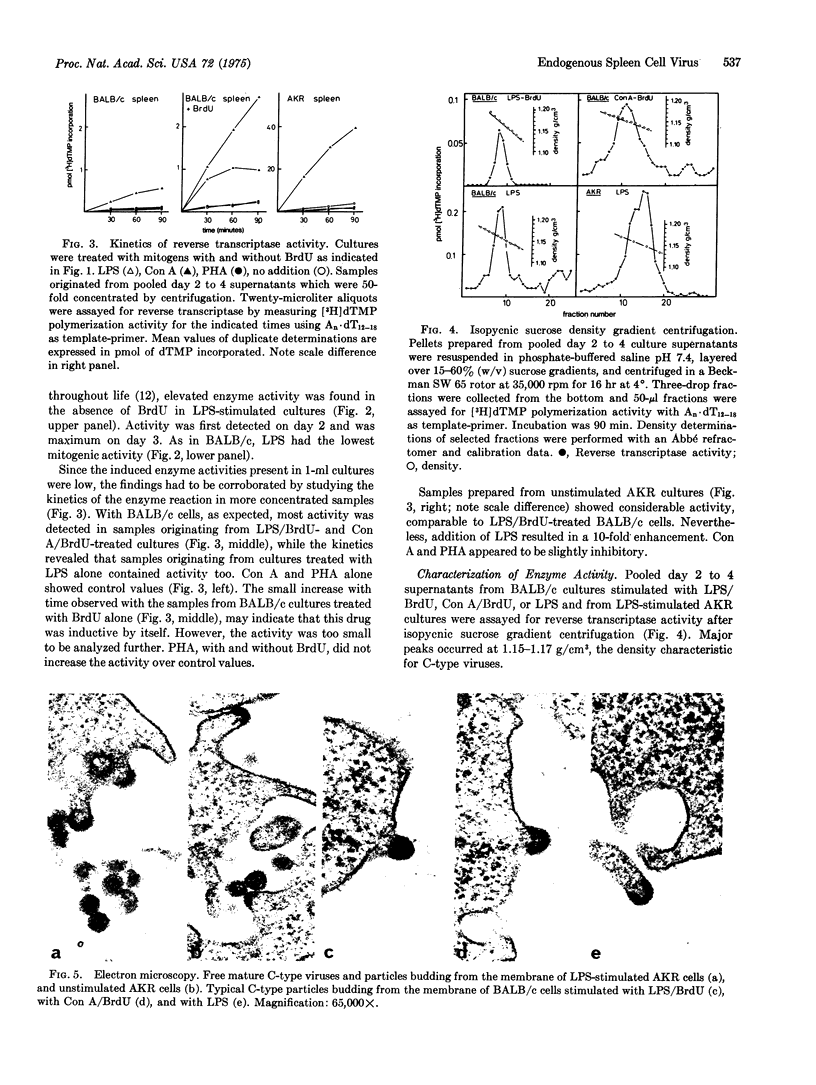
In short-term cultures of BALB/c spleen cells, treatment with a combination of 5-bromo-2'-deoxyuridine (BrdU) and either lipopolysaccharide W. Escherichia coli or concanavalin A resulted in release of C-type virus into the medium. Only lipopolysaccharide induced virus release when given alone. This could be potentiated by a combined treatment with BrdU. In contrast, phytohemagglutinin at mitogenic concentration had no effect with or without BrdU, suggesting that inducibility may vary between various mitogen-responsive spleen cell populations. In AKR mice, spontaneous virus release was detectable in nonstimulated spleen cell cultures. This could be potentiated by lipopolysaccharide, whereas no further increase occurred upon additional BrdU treatment. The induced viruses had C-type characteristics in that they contained reverse transcriptase that could be distinguished from cellular enzymes by template-primer preference experiments. Furthermore, the enzyme activities were particle-associated, banding in isopycnic sucrose gradients at 1.15-1.17 g/cm-3. The presence of C-type viruses was confirmed by electron microscopy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Phillips S. M., Solnik C., Black P. H., Schwartz R. S., Carpenter C. B. Activation of leukemia viruses by graft-versus-host and mixed lymphocyte reactions in vitro. Proc Natl Acad Sci U S A. 1972 May;69(5):1069–1072. doi: 10.1073/pnas.69.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonai P., Declève A., Kaplan H. S. Spontaneous induction of endogenous murine leukemia virus-related antigen expression during short-term in vitro incubation of mouse lymphocytes. Proc Natl Acad Sci U S A. 1974 May;71(5):2008–2012. doi: 10.1073/pnas.71.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Smoler D. F., Baltimore D. Terminal deoxynucleotidyl transferase in a case of childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1973 Feb;70(2):521–525. doi: 10.1073/pnas.70.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C. Structural proteins of Rauscher leukemia virus and Harvey sarcoma virus. Virology. 1972 Jan;47(1):1–7. doi: 10.1016/0042-6822(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Schumann G., Schnebli H. P., Dukor P. Selective stimulation of mouse lymphocyte populations by lectins. Int Arch Allergy Appl Immunol. 1973;45(3):331–340. doi: 10.1159/000231051. [DOI] [PubMed] [Google Scholar]
- Teich N., Lowy D. R., Hartley J. W., Rowe W. P. Studies of the mechanism of induction of infectious murine leukemia virus from AKR mouse embryo cell lines by 5-iododeoxyuridine and 5-bromodeoxyuridine. Virology. 1973 Jan;51(1):163–173. doi: 10.1016/0042-6822(73)90376-0. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]



