Abstract
Fatty acid spin labels were used to measure the inherent flexibility of the lipid acyl chains in intact cell membranes of 3T3 mouse fibroblasts and several varieties of transformed 3T3 cells. No significant differences in inherent lipid flexibility were detected in the normal and transformed mouse fibroblasts. These results are compared with similar results obtained earlier for chick embryo fibroblasts. For the cells studied, a difference in the mobility of membrane glycoproteins of normal and transformed fibroblasts has either been demonstrated directly or inferred from differential agglutinability by other investigations. Therefore, a basis for correlating glycoprotein mobility and lipid motion in these cells was not found.
Full text
PDF

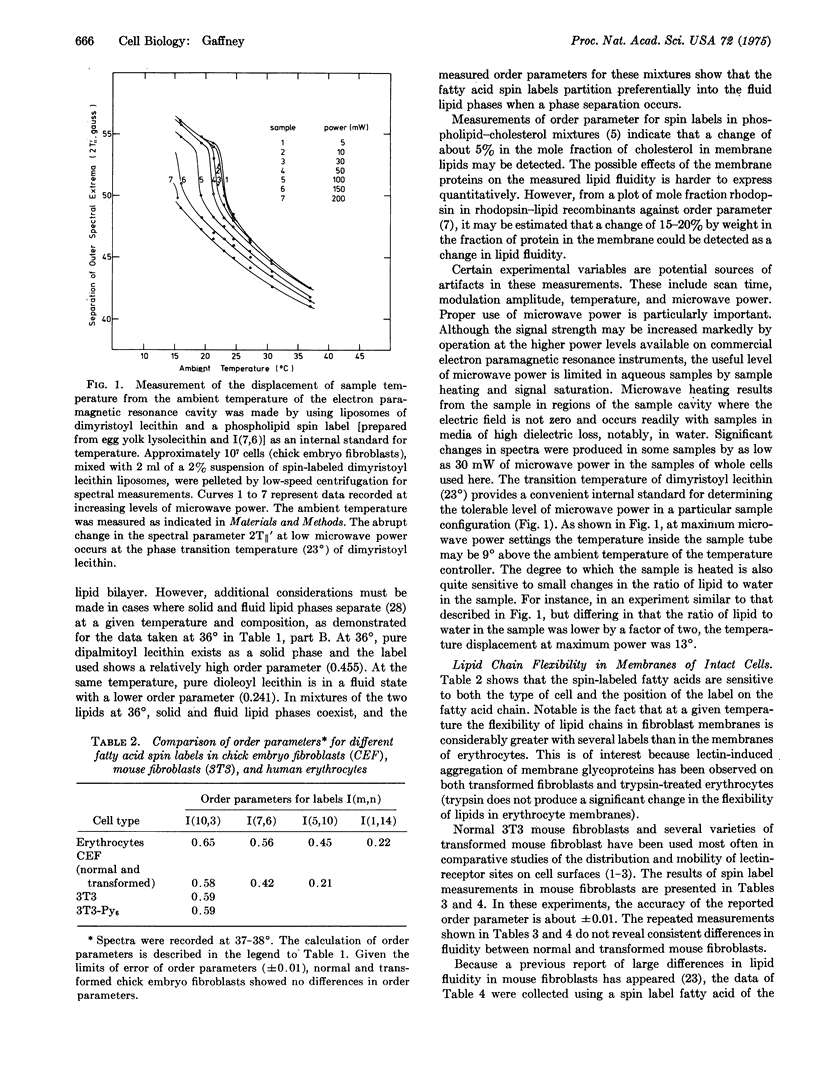
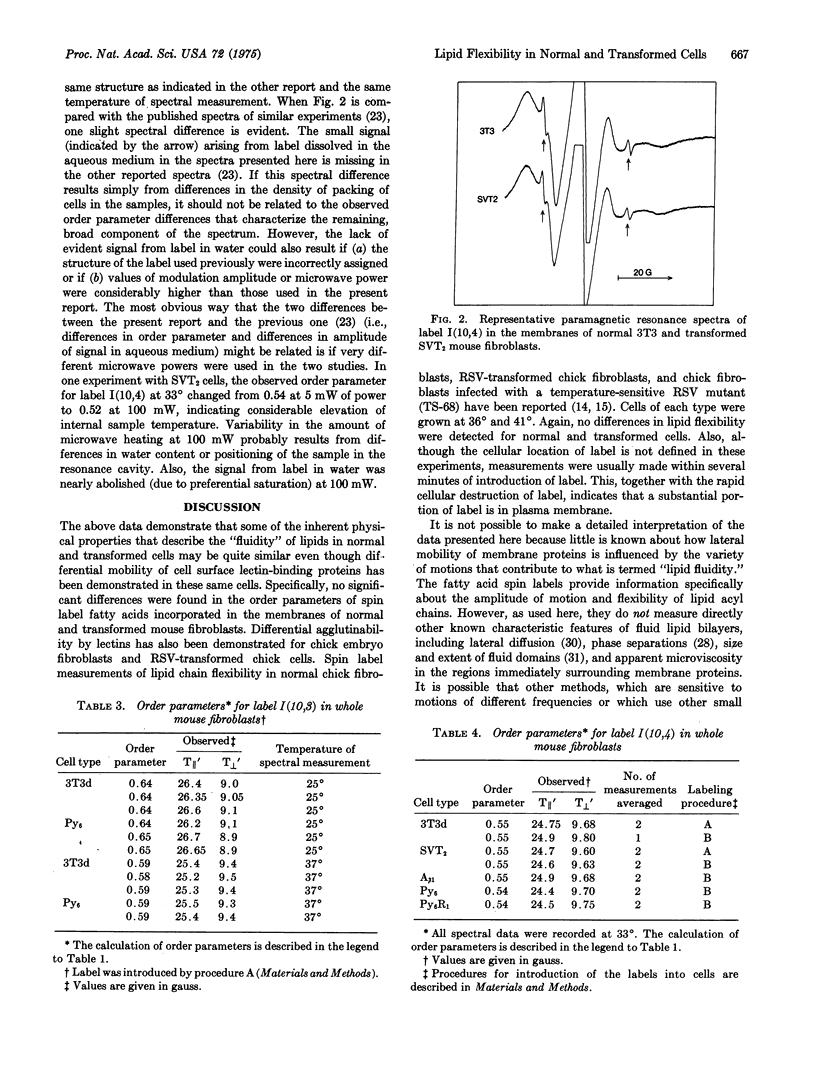

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett R. E., Furcht L. T., Scott R. E. Differences in membrane fluidity and structure in contact-inhibited and transformed cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1992–1994. doi: 10.1073/pnas.71.5.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. F., Hatten M. E., Burger M. M. Membrane fatty acid replacements and their effect on growth and lectin-induced agglutinability. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3115–3119. doi: 10.1073/pnas.71.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Metcalfe J. C., Metcalfe S. M., McConnell H. M. The interaction of small molecules with spin-labelled erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):415–427. doi: 10.1016/0005-2736(70)90219-1. [DOI] [PubMed] [Google Scholar]
- Huestis W. H., McConnell H. M. A functional acetylcholine receptor in the human erythrocyte. Biochem Biophys Res Commun. 1974 Apr 8;57(3):726–732. doi: 10.1016/0006-291x(74)90606-8. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- Kapeller M., Doljanski F. Agglutination of normal and rous sarcoma virus-transformed chick embryo cells by concanavalin A and wheat germ agglutinin. Nat New Biol. 1972 Feb 9;235(58):184–185. doi: 10.1038/newbio235184a0. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Kury P. G., Ramwell P. W., McConnell H. M. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun. 1974 Jan 23;56(2):478–483. doi: 10.1016/0006-291x(74)90867-5. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Sheppard J. R. Agglutinability by plant lectins increases after RNA virus transformation. Virology. 1972 Jul;49(1):339–341. doi: 10.1016/s0042-6822(72)80041-2. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Wright K. L., McFarland B. G. The fraction of the lipid in a biological membrane that is in a fluid state: a spin label assay. Biochem Biophys Res Commun. 1972 Apr 14;47(1):273–281. doi: 10.1016/s0006-291x(72)80039-1. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Kletzien R., Miller K. The isolation and characterization of plasma membrane from cultured cells. I. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted chick embryo fibroblasts. Biochim Biophys Acta. 1971 Dec 3;249(2):419–434. doi: 10.1016/0005-2736(71)90120-9. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Reich E. Lipid studies of Rous sarcoma virus and host cell membranes. Virology. 1972 Nov;50(2):550–557. doi: 10.1016/0042-6822(72)90406-0. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Seelig J., Hasselbach W. A spin label study of sarcoplasmic vesicles. Eur J Biochem. 1971 Jul 15;21(1):17–21. doi: 10.1111/j.1432-1033.1971.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Trudell J. R., Hubbell W. L., Cohen E. N. The effect of two inhalation anesthetics on the order of spin-labeled phospholipid vesicles. Biochim Biophys Acta. 1973 Jan 26;291(2):321–327. doi: 10.1016/0005-2736(73)90485-9. [DOI] [PubMed] [Google Scholar]
- Yau T. M., Weber M. J. Changes in acyl group composition of phospholipids from chicken embryonic fibroblasts after transformation by Rous sarcoma virus. Biochem Biophys Res Commun. 1972 Oct 6;49(1):114–120. doi: 10.1016/0006-291x(72)90016-2. [DOI] [PubMed] [Google Scholar]


