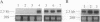Abstract
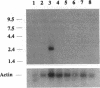
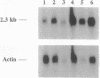
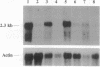
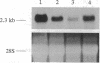
We have identified the nucleotide sequence of the cDNA encoding the human counterpart of the mouse gene Plk (polo-like kinase). The sequence of the human gene, PLK, predicts a serine/threonine kinase of 603 aa. Expression of PLK mRNA appeared to be strongly correlated with the mitotic activity of cells. Resting peripheral lymphocytes did not express the gene at all. When primary T cells were activated by phytohemagglutinin, a high level of PLK transcripts resulted within 2-3 days. In some cases, addition of interleukin 2 to these cells increased the expression of PLK mRNA further. In contrast, primary cultures of human peripheral macrophages, which were not dividing under the culture conditions applied, showed very little or no PLK mRNA. Stimulation of these cells by bacterial lipopolysaccharide, an inducer of several cytokines in macrophages, totally abrogated the expression of PLK mRNA. In line with a function of PLK mRNA expression in mitotically active cells is our finding that six immortalized cell lines examined expressed the gene. In A-431 epidermoid carcinoma cells this expression was down-regulated by serum starvation and enhanced after serum was added again. Tumors of various origin (lung, colon, stomach, smooth muscle, and esophagus as well as non-Hodgkin lymphomas) expressed high levels of PLK transcripts in about 80% of the samples studied, whereas PLK mRNA was absent in surrounding tissue, except for colon. The only normal tissues where PLK mRNA expression was observed were colon and placenta, both known to be mitotically active. No PLK transcripts were found in normal adult lung, brain, heart, liver, kidney, skeletal muscle, and pancreas. In Northern blot experiments with RNA from lymphocytes which were treated with phytohemagglutinin and cycloheximide, PLK transcripts were not detectable, suggesting that PLK is not an early growth-response gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuninger A., Holtrich U., Strebhardt K., Rübsamen-Waigmann H. Isolation and characterization of a human gene that encodes a new subclass of protein tyrosine kinases. Gene. 1992 Jan 15;110(2):205–211. doi: 10.1016/0378-1119(92)90649-a. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Böhme B., Holtrich U., Wolf G., Luzius H., Grzeschik K. H., Strebhardt K., Rübsamen-Waigmann H. PCR mediated detection of a new human receptor-tyrosine-kinase, HEK 2. Oncogene. 1993 Oct;8(10):2857–2862. [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clay F. J., McEwen S. J., Bertoncello I., Wilks A. F., Dunn A. R. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Enrietto P. J., Payne L. N., Hayman M. J. A recovered avian myelocytomatosis virus that induces lymphomas in chickens: pathogenic properties and their molecular basis. Cell. 1983 Dec;35(2 Pt 1):369–379. doi: 10.1016/0092-8674(83)90170-8. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Holtrich U., Bräuninger A., Strebhardt K., Rübsamen-Waigmann H. Two additional protein-tyrosine kinases expressed in human lung: fourth member of the fibroblast growth factor receptor family and an intracellular protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10411–10415. doi: 10.1073/pnas.88.23.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn T., Holtrich U., Bräuninger A., Böhme B., Wolf G., Rübsamen-Waigmann H., Strebhardt K. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993 Dec;8(12):3433–3440. [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov E. A., Levitina T. L., Gusak N. M. The primary structure of baculovirus inclusion body proteins. Evolution and structure-function aspects. Curr Top Microbiol Immunol. 1986;131:135–164. doi: 10.1007/978-3-642-71589-1_8. [DOI] [PubMed] [Google Scholar]
- Kubbies M., Schindler D., Hoehn H., Rabinovitch P. S. BrdU-Hoechst flow cytometry reveals regulation of human lymphocyte growth by donor-age-related growth fraction and transition rate. J Cell Physiol. 1985 Nov;125(2):229–234. doi: 10.1002/jcp.1041250209. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991 Dec;5(12A):2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Roberts T. M. Cell biology. A signal chain of events. Nature. 1992 Dec 10;360(6404):534–535. doi: 10.1038/360534a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Shimosato Y., Nakajima T., Hirohashi S., Morinaga S., Terasaki T., Yamaguchi K., Saijo N., Suemasu K. Biological, pathological and clinical features of small cell lung cancer. Cancer Lett. 1986 Dec;33(3):241–258. doi: 10.1016/0304-3835(86)90064-9. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Neel B. G., Stevens R., Evett G., Erikson R. L. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992 Sep;12(9):4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Dodson G. S., Rubin G. M. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993 Apr 9;73(1):169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Sunkel C. E., Glover D. M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988 Jan;89(Pt 1):25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993 Apr 9;73(1):5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The interleukin-2 receptor. J Biol Chem. 1991 Feb 15;266(5):2681–2684. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zhou J., Doorbar J., Sun X. Y., Crawford L. V., McLean C. S., Frazer I. H. Identification of the nuclear localization signal of human papillomavirus type 16 L1 protein. Virology. 1991 Dec;185(2):625–632. doi: 10.1016/0042-6822(91)90533-h. [DOI] [PubMed] [Google Scholar]
- von Briesen H., Andreesen R., Rübsamen-Waigmann H. Systematic classification of HIV biological subtypes on lymphocytes and monocytes/macrophages. Virology. 1990 Oct;178(2):597–602. doi: 10.1016/0042-6822(90)90361-t. [DOI] [PubMed] [Google Scholar]