Abstract
Respiratory infection of young infants results in increased morbidity and mortality compared to infection of adults. In spite of the significance of this health issue, our understanding of the immune response elicited in infants, especially in the respiratory tract is highly limited. We developed a nonhuman primate model to probe the virus-specific antibody response in infants following infection with influenza virus. Infection of infants resulted in more pulmonary damage and higher viral loads compared to adults. While the systemic IgG antibody response was similar in infant and adult animals, the response in the upper respiratory tract of the infant was compromised. This lower response was associated with an increased prevalence of Treg cells and low levels of BALT. These data suggest a defect in the ability to produce effective virus-specific antibody responses at the local infection site is a contributor to increased pulmonary damage in the at-risk infant population.
Keywords: neonatal immunity, respiratory infection, influenza virus, antibody, bronchus associated lymphoid tissue, lung pathology
INTRODUCTION
Respiratory infections are one of the leading causes of morbidity and mortality throughout the world. These infections, of which respiratory syncytial virus (RSV), rhinovirus (RV) and influenza virus are among the most prevalent [1], are particularly problematic for infants, resulting in increased morbidity and mortality compared to older children and adults. There are an estimated 11.9 million episodes of severe acute lower respiratory tract infection (ALRI) in young children each year [2]. Children under one year of age account for 6.4 million instances of severe ALRI and nearly 3 million of these cases are considered very severe [2]. Together these findings demonstrate the extreme susceptibility of the newborn to disease caused by respiratory pathogens.
As noted above, influenza virus is a significant health concern in infants. Not surprisingly, infection with influenza virus accounts for the greatest number of Emergency Department visits among children aged 6–23 months [3]. Further, infants younger than 6 months of age are particularly vulnerable to developing severe disease, with the highest risk of death occurring in the first year of life [4]. Disease states associated with influenza infection in children are varied and include otitis media, pneumonia, myositis, and laryngotracheobronchitis (croup). The last is restricted primarily to children less than 1 year of age and can be life threatening.
Currently, treatment of influenza relies on the use of antiviral drugs. Only one (oseltamivir) of the two currently recommended drugs is approved for infants <6 months of age and it must be administered within 48h of symptoms onset to realize benefit from treatment. Thus, there are limited therapeutics available for infants. Further, while an effective influenza vaccine is available, it is not approved for use in children less than 6 months of age. While this may reflect some safety concerns, a principal factor is the reduced ability of the neonatal immune system to respond to the influenza vaccine [5, 6]. These issues make clear the need for an improved understanding of the immune response generated in infants following respiratory infection with influenza virus.
Antibody plays an important role in the clearance of influenza virus (e.g. [7–9]). Studies in animal models show that the absence of B cells results in a 50–100 fold increase in susceptibility to lethal influenza [8]. Generation of an optimal antibody response relies on the presence of CD4+ T cells [10], with Th1 cells exhibiting increased effectiveness compared to Th2 cells [11–13]. CD4+ T cells from infants have been reported to exhibit a propensity for differentiation into Th2 cells [14, 15]. In addition to alteration in effector function, T cells from neonates exhibit decreased sensitivity to TCR engagement [16–18] and thus it is likely that they are less efficiently activated following infection. These aspects likely contribute to impaired CD4+ T cell mediated generation of high affinity, isotype switched antibody following infection of neonates.
An important mechanism through which antibodies contribute to the control of infection is neutralization, which limits virus spread. In addition, recent data suggest that antibodies can promote phagocytosis and/or antibody dependent cell-mediated cytotoxicity (ADCC) through a process that is highly dependent on alveolar macrophages [19]. Both IgG and IgA antibodies are believed to be important in the control of virus in the respiratory tract [20, 21]. Although antibodies to HA, NA, M and P can be detected, those specific for HA and NA glycoproteins are highly associated with protection [22] and the ability to neutralize virus [10].
Studies suggest that in general, vaccination or infection of infants results in an antibody response that is reduced in both the quantity and quality of antibody compared to adults (for review see [15]). Young infants infected with RSV or PIV fail to consistently develop serum or mucosal antibody [23, 24]. In humans, antibody responses following vaccination in young infants are largely IgM, with weak IgG responses during the first year of life [25]. Further, analysis of infants immunized with measles vaccine at 6 months of age revealed a defect in the generation of antibody compared to infants immunized at 9–12 months of age [26]. This effect was independent of the presence of maternal antibody. A similar finding was observed in infants that received the trivalent inactivated influenza vaccine (TIV). Administration of a single dose of TIV did not result in seroconversion [5] and a second TIV dose resulted in a protective titer rate of only 29–32% across H1N1 strains [5, 6]. These results show that young infants have impaired antibody responses, leaving them susceptible to severe disease.
The significant mortality and morbidity associated with respiratory virus infection in infants <6 months of age together with the absence of an effective vaccines demonstrates the importance of understanding the defects in the immune response in this at risk population. Progress in this area would be enhanced by a model that more closely mimics the human neonate with regard to physiology and immune system maturation/responsiveness. To this end, we have developed a model of influenza A virus (IAV) infection using the nonhuman primate (NHP) neonate in order to more fully elucidate the defects in the infant immune response following respiratory infection with virus. We believe that the nonhuman primate is an excellent model for studies of infant respiratory infection with regard to both physiology and immune system development. Specifically, the lungs of NHP are more similar in structure to the human than is the mouse lung [27]. In addition, the immune system does not display the reduced maturity at birth that is found in mice [15, 28]. Finally, the distribution and function of innate immune sensors in NHP versus mice are more comparable to humans [29].
We show that neonate African green monkeys (AGM) develop more severe lung damage following infection compared to adult animals. This was associated with lower levels of virus-specific IgG antibody in the upper respiratory tract. Surprisingly, systemic influenza-specific IgG levels were relatively similar. The lower antibody level in the respiratory tract was associated with an absence of organized BALT in infants, suggesting a defect in the local immune environment may contribute significantly to influenza-mediated diseases in neonates. Interestingly neonates also exhibited higher frequencies of T regulatory cells which may contribute to weakened immune responses.
MATERIALS AND METHODS
Ethics Statement
All research performed using animals in this study complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee. All studies were approved by the Wake Forest University Animal Care and Use Committee.
Influenza A/PR/8/34 (H1N1)
Virus stocks were grown and titered in fertilized chicken eggs (median egg infectious dose (EID50)) essentially as described previously [30]. Stocks were diluted in PBS, flash frozen, and stored at −80°C.
Infection and sampling
Adult African green monkeys (6–9 years old) were sedated with 10–15 mg/kg of ketamine. Infants (6–10d old) were sedated with 2–5% inhalant isoflurane. Adults received 5×109 EID50 of PR8 and infants received 1×109 EID50 (One of the infants received 1×108 EID50). The dose was delivered equally between the intranasal (i.n.) and intratracheal (i.t.) routes. Adults received 1.0 ml i.t. and 1.0 ml i.n. (0.5 ml per nostril). Infants received 0.25 ml i.t. and 0.25 mL i.n. (0.125 ml per nostril). On each sampling day, adults and infants were sedated. Blood was collected in sodium heparin tubes by venipuncture on d8 and d14 postinfection. Plasma was obtained by centrifugation and PBMC subsequently isolated using Isolymph. Tracheal washes were performed on sedated animals on d2, 8 and 14 postinfection by inserting an endotracheal tube into the trachea, instilling sterile PBS and aspirating back. In adult animals, 15 ml of PBS was instilled. In infant animals, 1.0 ml was instilled. Due to the small volume of PBS used in the infants, 0.5mL of PBS was used to wash out the endotracheal tube. Samples were centrifuged to remove cellular material and BSA was added to a final concentration of 0.5%. Bronchial alveolar lavage (BAL) with PBS was performed at necropsy (d14) using 25ml for adults and 5 ml for infants. Samples were centrifuged to remove cellular material and BSA added to a final concentration of 0.5%.
Assessment of lung pathology
Lung was preserved in 10% neutral buffered formalin for at least 24 hours, trimmed, embedded in paraffin and processed routinely for histology. Sections were cut at 6μm and stained with hematoxylin and eosin. The slides were examined by light microscopy by an ACVP (American College of Veterinary Pathologists) board certified veterinary pathologist in a blinded fashion and evaluated for degree of inflammation and injury. Pathology scores are based on a summation of scores assigned for interstitial and alveolar inflammatory cell infiltration and edema, pneumocyte hyperplasia, and bronchial degeneration and necrosis. Each parameter was scored on a scale of 0 to 4 with higher numbers associated with increased severity.
Quantitation of viral load
Viral RNA was extracted from tracheal wash using QIAamp Viral RNA Mini Kit (Qiagen). cDNA was synthesized from mRNA by reverse transcription using Superscript III RT kit (Invitrogen) and random primers (Invitrogen). For viral quantification, RNA primer-probe sets specific for H1N1 were used (BEI Resources). RT-PCR (qRT-PCR) was performed using the Applied Biosystems 7500 real-time PCR system. Copy number was calculated based on the copy number present in the sample volume used for the RT-PCR (140 μl) and adjusting to the total volume used for the wash.
ELISA for detection of influenza-specific antibody
Nunc MaxiSorp Elisa plates were coated with 1 μg/well PR8 in sodium carbonate/bicarbonate coating buffer (pH 9.5). Plates were blocked with 1x Blocking Buffer (10x Blocking Buffer, Sigma) plus 2% Goat serum (Lampire Biologicals) and washed. The wash buffer used throughout the assay was PBS with 0.1% Tween 20. Plasma or respiratory samples were serially diluted in 1x Blocking Buffer. Wells without virus served as a negative control. Antibody specific for monkey IgG (Fitzgerald), IgM (LifeSpan Bioscience) or IgA (AbD Serotec) was used to detect bound plasma antibody. IgG and IgM detecting antibodies were directly conjugated to HRP. Anti-IgA antibody was biotinylated and was detected with Streptavidin-HRP. Plates were developed with 3,3′,5,5′-Tetramethylbenzidine dihydrochloride (Sigma) and read at 450nm on a BioTek Elx800 Absorbance Microplate Reader. Absorbance for each dilution was calculated by subtracting the OD value obtained for the corresponding non-virus coated wells. Threshold titer was defined as the value that reached 3 times the assay background, i.e. wells that received only sample diluent.
Neutralization assay
Heat-inactivated (56°C for 1 hour) samples were serially diluted in RPMI-1640 media supplemented with 2 mM L-glutamine, 1 mM Sodium pyruvate, 1x non-essential amino acids (NEAA), 100U/mL penicillin, 100 μg/mL streptomycin, 50 μM 2-ME, 10% FBS in a sterile 96-well flat bottom plate. 7.5×106 EID50 of PR8-GFP (kindly provided by Dr. Adolfo Garcia-Sastre [31]) was added to each well and incubated for 2 hours at 37°C and 5% CO2 to allow for antibody binding. 2×105 U937 cells were then added to each well and incubated overnight at 37°C. The next morning, samples were acquired on a BD FACSCalibur and analyzed with CellQuest Pro software (Becton Dickinson) to determine the percentage of U937 cells that were positive for GFP. Controls for each experiment consisted of U937 cells alone and U937 cells infected with the PR8-GFP virus in the absence of animal samples. Maximal %GFP was calculated for each experiment and non linear regression (Graphpad Prism) used to determine the dilution at which the 50% maximum PR8-GFP infected U937 cells was achieved.
Analysis of PBMC
Immune populations in PBMC obtained from animals at d14 postinfection were identified by staining with anti-CD3-biotin followed by avidin-FITC, anti-CD8α APC-H7, anti-CD8β PE, anti-CD4 PerCpCy5.5, anti-CD20 Pacific Blue, and anti-CD95 APC. Samples were acquired on a BD FacsCanto II and analyzed with Diva software (Becton Dickinson).
Treg analysis
Lung, spleen, and tracheobronchial lymph nodes (TBLN) were isolated at necropsy. Lungs were minced and incubated in the presence of collagenase D for 1h. Digested tissues were mechanically disrupted by passage through a 70 μm filter. Single cell suspensions from this process were layered over a ficoll gradient and centrifuged to recover live cells. TBLN and spleen were mechanically disrupted and RBC lysed using ACK. To identify Tregs, cells were stained with anti-CD4 antibody (Clone L200, BD Bioscience). Following washing cells were permeabilized using BioLegend FOXP3 Fix/Perm and FOXP3 Perm buffer (BioLegend) followed by incubation with anti-FoxP3 antibody (Clone 206D, BioLegend). Samples were acquired on a BD FacsCanto II and analyzed with Diva software (Becton Dickinson).
RESULTS
Infant African green monkeys exhibit more pulmonary damage and impaired viral clearance compared to adult animals
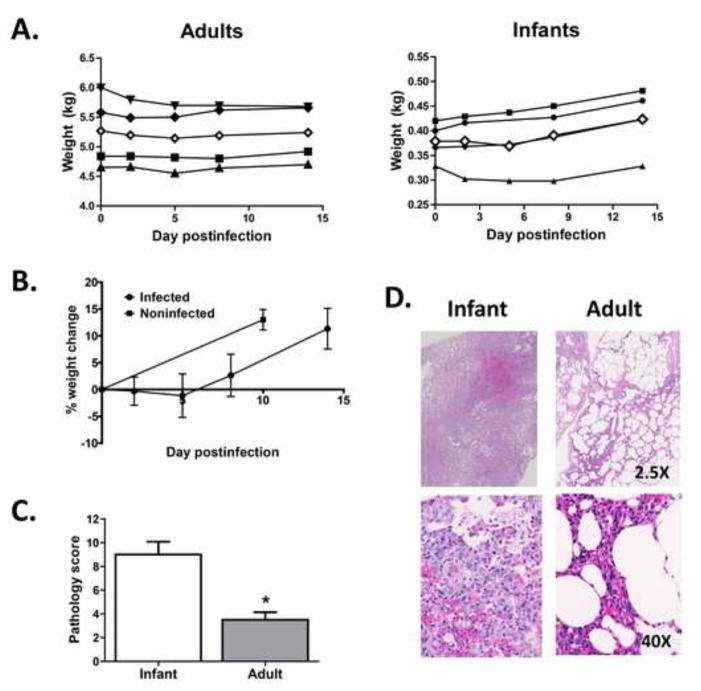
Four infant African green monkeys between 6 and 10 days of age (which approximates a 3–4 week old human infant) and four adults between 6 and 9 years of age (approximating a 24–36 year old human adult) were infected with the well characterized H1N1 influenza strain A/Puerto Rico/8/34 (PR8) by the combined intranasal and intratracheal routes. To control in part for the differences in lung volume between the two age groups, adult animals received 5-fold more virus as infection with the same inoculum dose would constitute a greater viral load per unit area of lung, complicating interpretation of the pathology changes caused by infection. Infants remained with the mothers throughout the infection as preliminary studies showed that mothers did not seroconvert (data not shown) and thus there was no evidence to suggest generation of maternal antibody that could be transferred to the infants. Mothers and adults used in the study had very low to undetectable levels of influenza-specific IgG (<1:200) and no detectable neutralizing antibody. Animals were monitored over time for changes in weight as well as signs of disease. All animals were normal with regard to activity and alertness. In general, adult animals did not exhibit significant changes in weight over the course of infection (Fig. 1A). Infants gained weight (Fig. 1A); however, this appeared to be at a lower rate than non-infected infants (Data shown for comparison are gathered from another study where infants where weighed at 4–6 days of age and again 10 days later) (Fig. 1B).
Figure 1. Infants infected with influenza virus exhibit significant increases in disease in the lung.
Infant or adult African Green monkeys were infected with IAV by the combined i.t. and i.n. routes. Three infants received 1×109 EID50 and one infant received 1×108 EID50 of PR8. Adults (4) each received 5×109 EID50. A. Weight over time for infant and adult animals. Closed symbols are individual animals, open symbol represents the average for the group. B. Average change in weight for infants over the course of the assay. Weight change over 10 days in a group of age-matched historical control animals is shown for comparison. C. On d14 p.i. animals were euthanized and lungs assessed for disease. Disease scores are based on a summation of scores assigned for interstitial and alveolar inflammatory cell infiltration and edema, pneumocyte hyperplasia, and bronchial degeneration and necrosis. Each parameter was scored on a scale of 0 to 4 with higher numbers associated with increased severity. Significance testing was performed using a Mann-Whitney test. * p=0.029. D. Representative sections of affected areas from the lungs of infant and adult animals (2.5X, upper panels and 40X, lower panels).
On d14 postinfection, animals were euthanized and lungs evaluated for signs of injury (alveolar wall thickness, hyperplasia, edema and bronchial necrosis). Each parameter was scored using a scale of 0–4 (with 0 being normal) in a blinded fashion by a veterinary pathologist. The values assigned for each parameter were summed to obtain a pathology score. The averaged pathology score from the infants was significantly increased compared to adults (p<0.03) (Fig. 1C). Representative photomicrographs from infant and adult lungs are shown in figure 1D. The infants typically had interstitial pneumonia, consistent with influenza. Alveolar walls were regularly thickened and alveolar spaces contained edema fluid and mixed type inflammatory cells, primarily macrophages and neutrophils. In contrast, adults exhibited only patchy interstitial pneumonia without edema fluid, and the infiltrating cells were predominantly lymphocytes and plasma cells.
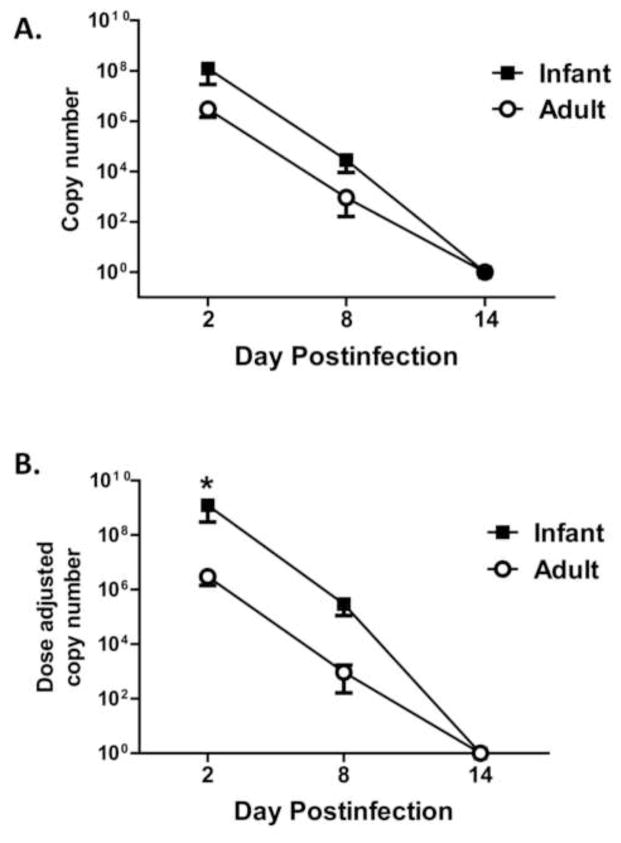
The severity of pulmonary injury in the infants suggested there may be a difference in viral load between the two groups. This was addressed by measuring the viral copy number in the tracheal washes on d2, d8, and d14 postinfection as determined by qRT-PCR (Fig. 2A). Infant animals had a 41-fold increase in virus in the trachea at d2 postinfection, despite that the dose of virus administered to the infants was five fold less (and for one animal 50-fold less) than in adults. To allow direct comparison of the relative viral load, values were normalized for the infectious dose delivered to the infants versus the adults (Fig. 2B). This analysis revealed that infants had on average a 413-fold increase in virus burden. Increased viral burden in the trachea of infants was also apparent at d8 p.i. The results of this analysis show increased virus burden in the airway of infants versus adults.
Figure 2. Infants have increased virus in the respiratory tract even in the face of a reduced inoculation dose.
Infected animals were sampled by tracheal lavage at the indicated days postinfection. Virus in lavage fluid was quantified by qRT-PCR and copy number determined. A. Averaged viral load in the trachea. B. Viral load normalized for differences in inoculation dose. Each infant animal copy number value was multiplied by 5 (or by 50 for the animal that received 1×108 EID50) to allow direct numerical comparison of the relative viral load in the infant versus adult animals. Significance at each timepoint was determined using a student’s t test. * p<0.05
Infants have a lower amount of influenza-specific IgG antibody in the respiratory tract compared to adults
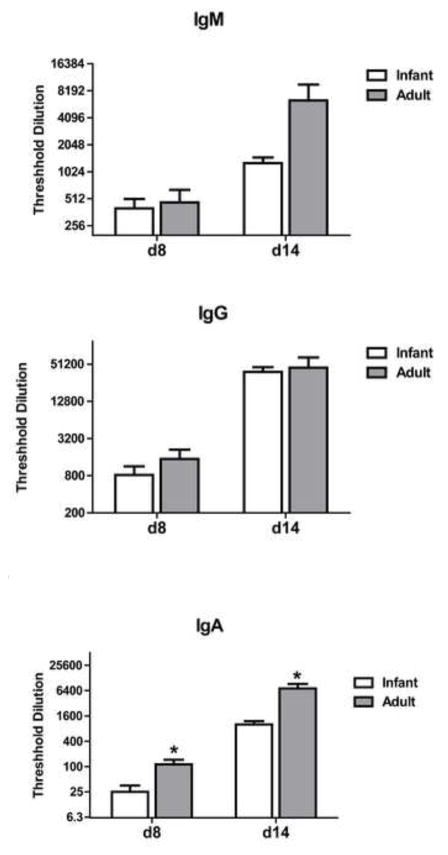
To assess the immune response elicited in adult and infant animals following infection, we determined the level of virus-specific antibody (IgM, IgG, IgA) in the trachea and lungs of infant and adult animals. Volumes utilized for the tracheal wash were 1.0 ml in infants and 15 ml in adults. The difference in volume used compensates for the calculated difference in size between infants and adults, allowing more direct comparison of the antibody titer between the two groups. Antibody in the trachea was analyzed at d8 and d14 p.i. Influenza-specific IgG was significantly lower in the trachea of infants compared to adults at d8 and d14 p.i. (Fig. 3). This difference was more pronounced at d8 (35-fold) compared to d14 (9.3-fold). The more pronounced difference in antibody level at d8 may suggest a delay in generation in the infant animals. Influenza-specific IgG in the lung at d14 was approximately twice as high on average in the adults compared to infants, although it was not significantly different (Fig. 3). (Antibody in the lung could not be assessed at the earlier timepoint.) There was no significant reduction in IgA in the lungs of infant animals as determined by analysis of the BAL (Fig. 3). Unfortunately limited sample obtained from the infants precluded determination of IgA in the trachea. On average, IgM was decreased in the infant animals, although this did not reach statistical significance (Fig. 3). Together these data demonstrate an impaired virus-specific IgG antibody response in the respiratory tract that is characterized by a delay in production as well as a lower quantity.
Figure 3. Respiratory tract influenza-specific IgG antibody levels are lower in infant animals.
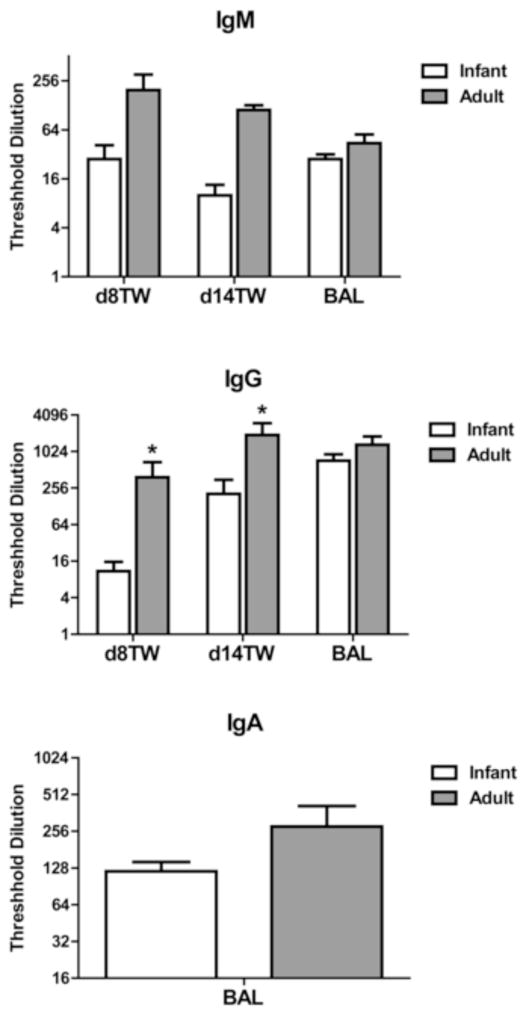
The amount of influenza-specific IgG, IgM, and IgA antibody in the tracheal wash (d8 and d14 p.i.) and/or BAL (d14 p.i.) was determined. IgA could not be assessed in tracheal washes due to limiting sample in the infants. Threshold dilution was defined as the dilution at which the influenza-specific OD reading (OD from influenza coated wells – OD from uncoated wells) reached three times the assay background. Significance at each timepoint was determined using a Mann Whitney test. * p≤0.05.
Adults have a higher level of neutralizing antibody in the trachea compared to infants
We next assessed the neutralizing potential of antibody present in the respiratory tract. For these studies, titrated concentrations of tracheal wash fluid or BAL were incubated with influenza virus that expresses GFP. Virus was then added to U937 indicator cells and cultured overnight, following which GFP expressing cells were quantified by flow cytometry. The neutralization titer was defined as the dilution of plasma that resulted in 50% inhibition of the percentage of GFP+ cells present in the absence of plasma (usually 80–90% of the cells). We chose this approach as it is a direct measure of the ability of antibody to prevent infection. However, to validate this approach in our hands we have compared results obtained to the standard HAI using plasma samples where we had adequate sample for this analysis (data not shown). We found that these two approaches led to similar conclusions, although the infectivity assay shown here was somewhat more sensitive. These analyses revealed a trend towards a higher level of neutralizing antibody at d8 and a significantly higher level of neutralizing antibody in the trachea of adult animals at d14 p.i. (Fig. 4). Although on average the level of neutralizing antibody in the BAL was higher in adults, it did not reach statistical significance (Fig. 4). Together with the data in figure 3, these findings support increases in both the quality and quantity of antibody present in the respiratory tract of adult compared to infant animals.
Figure 4. The neutralizing titer of trachea-derived influenza-specific antibody is lower in infant animals.
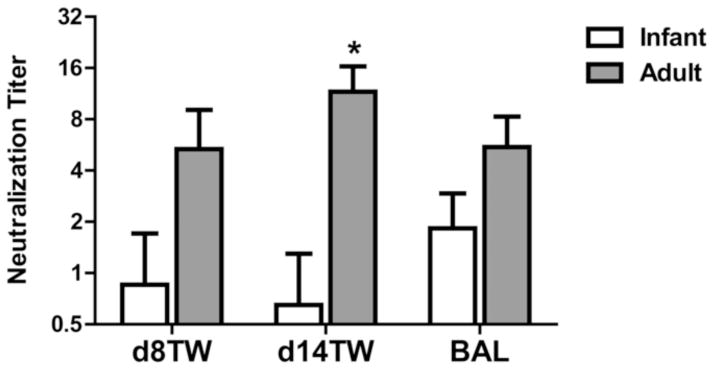
Neutralizing antibody titer in the tracheal wash and BAL of infected infant and adult animals was assessed at d8 and d14 p.i., respectively. Neutralization capacity was determined by inhibition of infectivity of a GFP expressing virus. The neutralization titer was defined as the dilution at which infectivity of the virus was inhibited by 50%. Significance at each timepoint was determined using a Mann Whitney test. * p≤0.05.
Systemic influenza-specific IgG antibody responses are similar in infants and adults
We next evaluated systemic antibody in the adult and infant animals as this is the most common approach for evaluating responses in humans. Influenza specific antibody in the plasma was measured at d8 and d14 postinfection. IgM levels at d8 were similar in adult and infant animals with a trend towards increased IgM in adults at d14 p.i (Fig. 5). No differences were found in the circulating level of influenza-specific IgG antibody between the two groups at either timepoint, although a significantly higher level of IgA was observed in adult animals at both d8 and d14 p.i. These data show that, in contrast to what was observed in the respiratory tract, neonates exhibited a robust systemic IgG response following infection with IAV that was similar to that observed in adults. To determine whether there was a difference in the quality of the antibody, neutralization capacity was assessed. As with IgG level, no significant differences in neutralizing titers were observed (Fig. 6).
Figure 5. Systemic influenza-specific IgA, but not IgG or IgM antibody levels, are higher in adult versus infant animals.
The amount of influenza-specific IgG, IgM, and IgA antibody in the plasma was determined at d8 and 14 postinfection. Threshold dilution was defined as the dilution at which the influenza-specific OD reading (OD from IAV coated wells – OD from uncoated wells) reached three times the assay background.
Figure 6. The neutralizing titer of systemic influenza-specific antibody is similar in adult and infant animals.
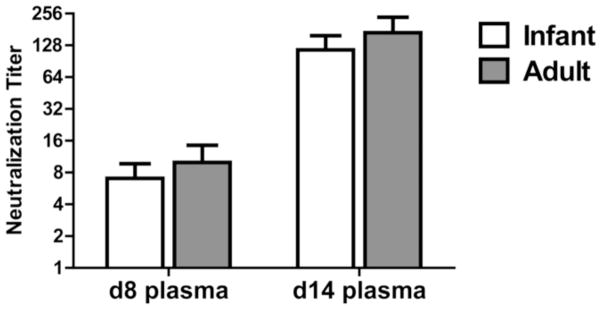
Neutralizing antibody titer in the plasma of infected infant and adult animals was assessed at d8 and d14 p.i. Neutralization capacity was determined by inhibition of infectivity of a GFP expressing influenza virus. The neutralization titer was defined as the dilution at which infectivity of the virus was inhibited by 50%. Significance at each timepoint was determined using a Mann Whitney test. * p≤0.05.
Infants and adults differ in PBMC populations at d14 p.i. To gain insights into the immune populations present in infant and adult animals following infection, the frequency of T and B cells in circulation was determined. While the percentage of T cells did not differ between infant and adult animals, there was a marked increase in B cells (Fig. 7A). Although the percentage of T cells was similar in adults and infants, there was a pronounced shift towards CD4+ cells in infants, perhaps suggestive of a reduced CD8+ T cell response in these animals. When CD95 was evaluated as a marker of activation, no differences were observed in the T cells subsets. In contrast, the percentage of B cells that expressed high levels of CD95 was significantly different between the two groups, with infants having a much lower proportion of B cells that had undergone activation.
Figure 7. The circulating populations of immune cells at d14 p.i. differ in adult versus infant animals.
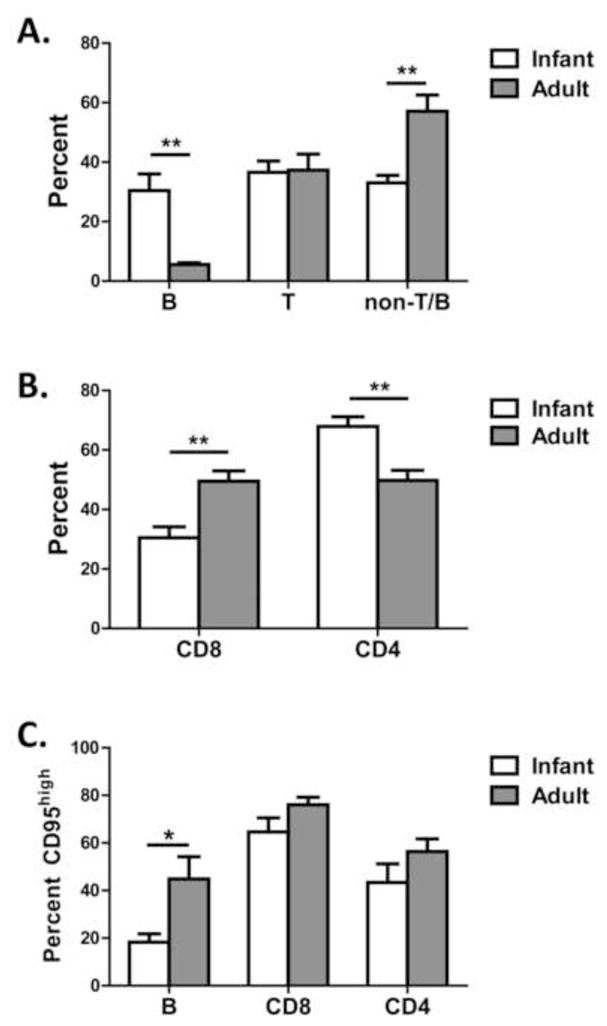
PBMC at d14 p.i. were assessed for the relative proportion of (A) T and B cells, (B) CD4 versus CD8 subsets, and (C) CD95high cells within B and T cell populations. *p≤0.05, **p≤0.01
Infants have less BALT compared to adults
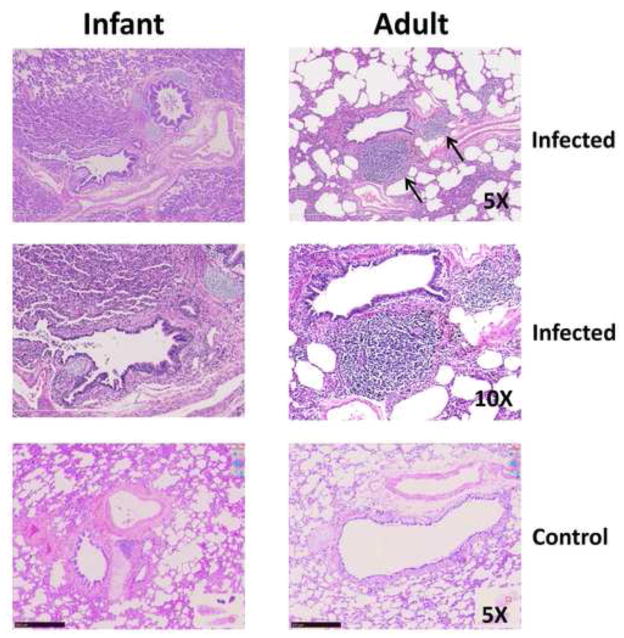
The disparity between the levels of antibody in the upper respiratory tract and circulation between adult and infant animals was unexpected. Antibody in the respiratory tract is a reflection of local production in the airway in addition to production in the draining lymph nodes. Several recent studies have reported tertiary lymphoid tissues, e.g. BALT, as a significant source of antibody following infection with influenza virus [32, 33]. We hypothesized that one possibility to explain the reduced antibody present in the infant animals in our study was a decrease in the induction of BALT in response to virus infection. Histological evaluation of the BALT in the infant animals consistently revealed a near absence of this structure, while it was considered robust in adults, as evidenced by prominent collections of lymphocytes situated around bronchi and bronchioles (Fig. 8). The presence of robust BALT structures in the adult animals is an infection related event, as analysis of non-infected age-matched controls revealed limited BALT (Fig. 8). The absence of BALT in infant animals is consistent with the lower level of antibody in the respiratory tract.
Figure 8. Infants infected with influenza virus lack detectable BALT.
Photomicrographs of lung from an infected and control infant (left column) and adult (right column) AGM. H and E staining are shown at 5X and 10X for the infected and 10X for the control animals. The infected adult has prominent round accumulations of lymphocytes adjacent to a bronchiole, structures absent around the bronchus (supported by cartilage) and the adjacent bronchiole. BALT is indicated by arrows.
Tregs are increased in infants
A previous study performed in mice suggested Tregs can suppress generation of BALT [34]. Thus, we assessed the presence of Treg in the TBLN and lung of adults and infants at necropsy (d14 postinfection). We found a significant increase in the representation of FoxP3+ cells within the CD4+ subset in the LN of infant animals compared to adults (Fig. 9A). Further, there was an increase in FoxP3+ cells in the lungs of infants, although the increase did not achieve statistical significance (Fig. 9B). To address whether the increased frequency of Tregs was the result of differences in the response to infection versus inherent differences in the immune system of adult and infant animals, the frequency of FoxP3+ cells within the CD4+ T cell population was assessed in the spleen of infected and non-infected adult and infant animals. No significant differences were found between the infected animals and their non-infected counterparts for either age group. However, a highly significant difference in the percentage of Tregs was observed, with infants having a 2.8 fold increase in the percentage of CD4+ T cells that express FoxP3. The higher number of Tregs could contribute to the reduced antibody response in infants through both suppression of BALT formation and inhibition of T and B cell responses.
Figure 9. T regulatory cells are increased in infants.
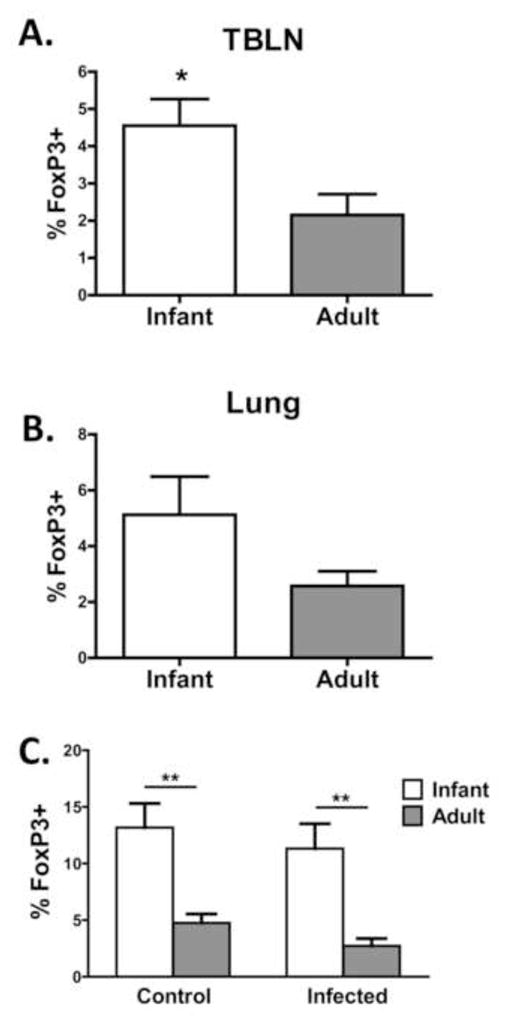
On d14 p.i., the tracheobronchial lymph nodes (TBLN) (A), lung (B) and spleen (C) were isolated and processed to yield single cell suspensions. Spleens were also isolated from non-infected animals (C). Cells were stained for CD4+ and FoxP3+. Averaged data shown are the percentage of FoxP3-expressing cells within the CD4+ population. Significance at each timepoint was determined using a student’s t test. *p≤0.05, **p≤0.01.
DISCUSSION
We investigated the capacity for neonates to elicit an antibody response to IAV infection using a novel nonhuman primate model. We found respiratory infection with IAV resulted in increased viral load in the respiratory tract of infants compared to adults following inoculation. Infection of neonates was also associated with significantly greater pulmonary damage. Surprisingly, systemic levels of influenza-specific IgG were similar in infants and adults, although there was a reduction in IgA. In contrast, respiratory IgG was consistently lower in infants compared to adults. The reduced antibody in the respiratory tract was associated with an absence of organized BALT in the infants. The selective decrease in antibody in the airway suggests a defect in the local production of antibody may contribute to increased pulmonary damage in this population.
The generation of a robust antibody response is an important contributor to the clearance of influenza virus [7]. The vast majority of our understanding of efficacious influenza-specific antibody responses has come from studies in adult animals. These analyses have determined that IgG and IgA responses are capable of promoting protection/clearance [7–9,19, 35]. There is some indication that distinct isotypes may play different roles depending on the site, with IgA playing a more important role in the upper respiratory tract and IgG in the lung [21]. Whether this is the case in humans is not clear.
At present, to our knowledge, there is only a single study evaluating the antibody response in neonates following IAV infection. In a mouse model using 3 day old animals, 1 of 5 animals exhibited positive antibody titers at 13 days postinfection compared to 5 of 5 adults [36]. The vast majority of additional data available in relation to influenza-specific immune responses in infants is derived from studies of vaccination in humans. Trivalent inactivated influenza vaccine (TIV) in 3–5 months old infants did not result in seroconversion following initial administration, with the exception of one virus strain (A/Mississippi/11/85, which had a 40% conversion rate for reasons that are unknown) [5]. This low responsiveness was not due to inhibitory effects of maternal antibody. A second TIV dose resulted in a protective titer rate of approximately 29% across all strains evaluated. Not surprisingly, the conversion was directly correlated with age as older infants converted at a higher rate than younger infants [5]. In the second study, conversion was assessed following two doses of vaccine with a conversion rate of 32% for H1N1 and 47% for H3N2 strains [6]. Thus, the ability of infants to respond effectively to influenza vaccination is limited.
Our results showed that respiratory infection with IAV resulted in a significantly lower influenza virus-specific IgG response in the respiratory tract. The presence of antibody at local sites of infection, e.g. the lung, is the result of systemic antibody that enters the respiratory tract via circulation as well as the local production by respiratory tract associated lymphoid tissue. Pathogens entering the nasal passage initiate responses in the nasal associated lymphoid tissue (NALT). As they spread to the lower respiratory tract, they initiate immune activation in the tracheal/bronchial associated lymphoid tissue (BALT).
BALT is relatively absent in healthy humans and mice [37, 38], developing in response to inflammatory/infection derived signals [39–41]. BALT contains organized structures that are similar to lymph nodes in that they have defined T and B cell areas [32]. Germinal centers [32] form during the immune response resulting in production of high affinity antibody and long lived plasma cells. While much less studied than responses in the draining lymph node, recent data support an important contribution of BALT derived responses in the control of influenza virus infection [32, 42, 43]. In fact, responses generated in BALT tissue may be superior to those originating in the secondary lymphoid tissues as these immune responses are less pathogenic [32].
A number of cytokines and chemokines have been implicated in BALT development including lymphotoxin α, CXCL13, CCL19, and CCL21 (for review see [40]). Much remains to be learned with regard to the expression of these factors in the infant lung. It is tempting to speculate that reduced production of these mediators in infants contributes to impaired BALT formation. In support of this possibility, CXCL13 expression has been reported to be decreased in the lymph nodes of neonatal mice [44]. However, in contrast, analysis of lymphotoxin α production following in vitro stimulation of mononuclear cells showed similar production by neonate versus adult derived cells [45]. The production of and responsiveness to these regulators is an important area for further investigation in the context of the infant lung.
While infection and inflammatory mediators are known signals for the induction of BALT, the presence of Treg has been reported to inhibit this process [34]. This seems to be reliant on the ability of Tregs to enter into lymphoid tissues in a CCR7, L-selectin dependent manner [34]. Interestingly, similar to our study, the effect of Tregs on BALT formation was associated with changes in Tregs in lung draining lymph nodes as opposed to the lungs. The increase in Tregs in the tracheobronchial lymph nodes of the infant animals assessed in our study would be consistent with a model where Tregs contribute to the failure of these infants to form BALT following IAV infection. Although we did not see a statistically significant increase in Tregs in the lungs of infected infants, there was on average a higher percentage of Tregs among CD4+ T cells in the lungs. A limitation to our analyses as well as the previous study of lung cells [34] is the analysis of Treg was performed using total lung cells as opposed to BALT resident cells. Whether there are differences in Tregs within the BALT, similar to the draining lymph node, awaits further study. The increase in Tregs appears to be a property of the AGM infant immune system. To our knowledge, this is the first indication of an increased T regulatory population in non-human primate infants.
In summary, our data suggest that a contributor to the increased susceptibility of young infants to respiratory infection is an impaired immune response in the respiratory tract. Infants in our study had lower levels of IgG at this site, which was more prominent at early times following infection. The lower level of antibody in the upper respiratory tract of infants was in contrast to the systemic response, where IgG antibody was similar in adults and infants. This finding suggests analysis of circulating antibody levels may not be indicative of the antibody response in the infected tissue and therefore may not fully represent the immune response. In addition, we show that infants display a significantly higher frequency of T regulatory cells both prior to and following infection. This population has the capacity to strongly modulate both B and T cell responses following viral challenge. In closing, our data suggest an important contribution of locally produced antibody to the control of virus level and pathology in vivo and suggest approaches to increase the local immune response in the tissue would be beneficial in protecting infants from virus-mediated respiratory disease.
Influenza virus infection results in more disease in NHP neonates versus adults
Neonates have lower virus-specific antibody in the respiratory tract
Neonates exhibit an increased prevalence of T regulatory cells
Neonates are impaired in the formation of BALT following virus infection
Acknowledgments
We thank Dr. Adolfo Garcia-Sastre for provision of influenza virus expressing GFP. We express our gratitude to Dr. Matthew Jorgenson and the animal care staff at the Vervet Research Colony for help with administration and care of animals. The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Swine Influenza A (H1N1) Real-Time RT-PCR Assay, NR-15577. We acknowledge the Flow Cytometry and CVVVL Shared Resources supported by NCI CCSG P30CA012197. These studies were supported by NIH R01 AI098339 and a grant from the Wake Forest University Translational Science Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moisi JC, Roca A, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgeois FT, Valim C, Wei JC, McAdam AJ, Mandl KD. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics. 2006;118:e1–e8. doi: 10.1542/peds.2005-2248. [DOI] [PubMed] [Google Scholar]
- 4.Esposito S, Principi N. The rational use of influenza vaccines in healthy children and children with underlying conditions. Curr Opin Infect Dis. 2009;22:244–249. doi: 10.1097/QCO.0b013e32832a58e4. [DOI] [PubMed] [Google Scholar]
- 5.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–828. [PubMed] [Google Scholar]
- 6.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect Dis. 2008;197:1448–1454. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 10.Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, Friede M, Wood D. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008;26:4299–4303. doi: 10.1016/j.vaccine.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26:2350–2359. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 13.Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naderi N, Pourfathollah AA, Alimoghaddam K, Moazzeni SM. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clin Exp Med. 2009;9:29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 15.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 16.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci USA. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerici M, DePalma L, Roilides E, Baker R, Shearer GM. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J ClinInvest. 1993;91:2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, Di VV, Manzoli FA. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J Immunol. 1999;163:2416–2424. [PubMed] [Google Scholar]
- 19.Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, Wherry EJ. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013;9:e1003207. doi: 10.1371/journal.ppat.1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renegar KB, Small PA., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 22.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 23.Welliver RC, Kaul TN, Putnam TI, Sun M, Riddlesberger K, Ogra PL. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980;96:808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh K, Masters HB, Orr I, Chao RK, Barkin RM. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 25.Randolph DA. The neonatal adaptive immune system. NeoReviews. 2005;6:e454–e462. [Google Scholar]
- 26.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 27.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makori N, Tarantal AF, Lu FX, Rourke T, Marthas ML, McChesney MB, Hendrickx AG, Miller CJ. Functional and morphological development of lymphoid tissues and immune regulatory and effector function in rhesus monkeys: cytokine-secreting cells, immunoglobulin-secreting cells, and CD5(+) B-1 cells appear early in fetal development. Clin Diagn Lab Immunol. 2003;10:140–153. doi: 10.1128/CDLI.10.1.140-153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Klimov A, Balish A, Veguilla V, Sun H, Schiffer J, Lu X, Katz JM, Hancock K. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Method Mol Biol. 2012;865:25–51. doi: 10.1007/978-1-61779-621-0_3. [DOI] [PubMed] [Google Scholar]
- 31.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 33.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuman PD, Ayoub EM, Small PA., Jr Influenza infection in the infant mouse. Pediatr Res. 1983;17:338–343. doi: 10.1203/00006450-198305000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1–8. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]
- 38.Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- 39.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. AdvImmunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010;3:537–544. doi: 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 41.Gould SJ, Isaacson PG. Bronchus-associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol. 1993;169:229–234. doi: 10.1002/path.1711690209. [DOI] [PubMed] [Google Scholar]
- 42.Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, Harmsen AG. Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat Res Biol. 2013;11:196–202. doi: 10.1089/lrb.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norton EB, Clements JD, Voss TG, Cardenas-Freytag L. Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model. J Virol. 2010;84:2983–2995. doi: 10.1128/JVI.01805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pihlgren M, Tougne C, Bozzotti P, Fulurija A, Duchosal MA, Lambert PH, Siegrist CA. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J Immunol. 2003;170:2824–2832. doi: 10.4049/jimmunol.170.6.2824. [DOI] [PubMed] [Google Scholar]
- 45.English BK, Burchett SK, English JD, Ammann AJ, Wara DW, Wilson CB. Production of lymphotoxin and tumor necrosis factor by human neonatal mononuclear cells. Pediatr Res. 1988;24:717–722. doi: 10.1203/00006450-198812000-00014. [DOI] [PubMed] [Google Scholar]