Abstract
Candida is an opportunistic fungal pathogen that colonizes the mucosal tract of humans. Pathogenic infection occurs in the presence of conditions causing perturbations to the commensal microbiota or host immunity. Early innate immune responses by the epithelium, including antimicrobial peptides (AMPs) and cytokines, are critical for protection against overgrowth. Reduced salivary AMP levels are associated with oral Candida infection and certain AMPs, including human beta-defensins 1 - 3, have direct fungicidal activity. Here we demonstrate that murine β-Defensin 1 (mBD1) is important for control of early mucosal Candida infection and plays a critical role in the induction of innate inflammatory mediators. Mice deficient in mBD1 exhibit elevated oral and systemic fungal burdens as compared to wild-type mice. Neutrophil infiltration to the sites of mucosal Candida invasion, an important step in limiting fungal infection, is significantly reduced in mBD1 deficient mice. These mice also exhibit defects in the expression of other antimicrobial peptides, including mBD2 and mBD4, which may have direct anti-Candida activity. We also show that mBD1 deficiency impacts the production of important anti-fungal inflammatory mediators including IL-1β, IL-6, KC, and IL-17. Collectively, these studies demonstrate a role for the mBD1 peptide in early control of Candida infection in a murine model of mucosal candidiasis, as well as on the modulation of host immunity through augmentation of leukocyte infiltration and inflammatory gene induction.
Keywords: Candida albicans, fungal, immunity, inflammasome, beta-defensins, mucosal
Introduction
Human fungal infections have dramatically increased in recent years, representing a major threat to public health worldwide. Candida albicans is the most common cause of human fungal infections and unlike many pathogens, Candida can exist as a common colonizer of mucosal sites. Overgrowth can occur during states of compromised host immunity, including extremes of age, steroid treatment, and AIDS, or when normal microbiota is pertubated such as with antibiotic use, leading to clinical infection. Infection with Candida is normally superficial and limited to mucosal surfaces; however, disseminated candidiasis can result in significant mortality (1, 2). The innate immune system has been an area of great research interest in fungal immunology, and its functions include physical impedance of infection, release of antifungal agents in mucosal secretions, and the infiltration/activation of blood leukocytes. Much work has been done on identifying the immune receptors responsible for fungal recognition, as well as characterizing the role of cytokines and other innate mediators in the development of a protective immune response. The role of epithelial cells in the initiation of innate responses to mucosal fungal infection is less well understood. Traditionally considered as a physical barrier preventing infection, recent evidence has demonstrated that epithelial cells play a very active role in the early immune response to pathogens. Release of inflammatory mediators from epithelial cells is a critical step for the generation of protective host responses including the recruitment of inflammatory cells as well as the generation of direct anti-microbial factors. In addition to the release of cytokines, including potent chemotactic factors, epithelial cells also release small anti-microbial peptides (AMPs) which are capable of exerting direct microbicidal effects. AMPs comprise a wide range of small peptides and can either be anionic or cationic. There are four major classes of AMPs: 1) Small anionic peptides which require zinc as a cofactor, 2) small α-helical cationic peptides (<40 amino acids) which lack cysteines, 3) linear cationic peptides rich in proline or tryptophan but lacking in cysteines, and 4) defensins, which are characterized by the presence beta-sheets stabilized by two disulfide bonds (3). Recent research suggests that the role of these bonds may extend beyond simple structural stabilization. For example, human beta defensin 1 (hBD1) was previously described as having low antimicrobial activity, yet was found to be a potent antimicrobial peptide after reduction of both disulfide bonds (4). Another recent report demonstrated that recombinant murine beta defensin 1 (mBD1), likely lacking disulfide bonds due to production in E. coli, had candidacidal activity through inhibition of growth and germ-tube formation which resulted in cell death (5). The mechanism of the antimicrobial activity of β-defensins remains somewhat controversial. A common characteristic of AMPs is the presence of regions enriched with charged amino acids, which are thought to be the basis for antimicrobial activity. Many reports have found the antimicrobial activity of β-defensins is salt sensitive. Changes in salinity modifies electrostatic interactions between polar/charged amino acids (6); therefore, the salt-sensitivity of β-defensins emphasizes the importance of cationic residues in mediating fungal activity (7-9). mBD3 was recently shown to induce microbial death through pore formation and membrane perforation; however, other reports have found that the anti-Candida activity of hBD2 and hBD3 was not dependent on membrane disruption demonstrating potentially divergent mechanisms of antimicrobial activity in humans and mice (8, 10, 11).
The roles of β-defensins in mediating pathogenic death are well documented but their function in the initiation of immune responses remains largely unknown. Mice represent an excellent system to assess the impact of defensins on induction of immune responses. Orthologs of human β-defensins (hBD) have been identified in mice including mBD1, which is homologous to hBD1 (12-15). Murine beta-defensin 1, mBD1 (DefB1), was first described as a salt-sensitive AMP present in the epithelium of the lung and urinary tract and found to be critical for control of Haemophilus influenzae virus infection (12, 16). Decreased salivary levels of antimicrobial peptides are commonly observed in patients suffering from oral candidiasis (17). It was recently discovered that recombinant mBD1 has direct fungicidal activity via inhibition of growth and germ tube generation (5). In another study, treatment of Candida infected mice with a non-peptide mimetic of mBD1 was shown to substantially reduce fungal burdens in the oral cavity, though the impact on innate immune activation was not studied (18). Other murine defensins have also been shown to have antifungal properties including mBD3 which induces perforation of the microbial cell wall, resulting in cell lysis and can amplify the antifungal properties of amphotericin (19). Human BD1, −2 and −3 have also been identified to have microbicidal activity against Candida (11, 20); and a recent study demonstrated more potent antimicrobial activity of hBD1 when reduced by thioredoxin in epithelium (4).
In the current study, the role of mBD1 during mucosal Candida infection was assessed. After oral infection with Candida albicans, mBD1 deficient mice exhibited increased mucosal and systemic fungal burdens during early (up to 14 days) infection with Candida. We also observed impaired upregulation of mucosal antimicrobial peptides, inflammatory cytokines and chemokines in the mBD1 deficient mice compared to wild-type mice following infection. Finally, we observed a profound defect in neutrophil infiltration to the mucosal sites of Candida infection. These studies identify a novel role for mBD1 in the induction of inflammatory cytokines, recruitment of inflammatory cells and early protection from mucosal fungal infection. Future studies on the role of β-Defensin 1 in human anti-fungal immunity may identify BD1 as a therapeutic target for individuals susceptible to Candida infection.
Materials and Methods
Animals
C57Bl/6 mice were purchased from Jackson Laboratories. DEFB1−/− mice were generated by JM Wilson (16). Mice were housed in HEPA filter-covered microisolator cages in ventilated racks. All animal were housed in AAALAC accredited facilities at Case Western Reserve University School of Medicine under approved protocols from the Institutional Animal Care and Use Committee.
Fungal strains
Candida albicans strain GDH2346 (NCYC 1467), a clinical strain originally isolated from a patient with denture stomatitis, was maintained on Sabouraud Dextrose (SD) agar. For use in the OPC infection model, yeast were grown for 16 h in SD broth, pelleted at 3000 rpm for 5 min, and washed 2X with sterile PBS, and diluted to a density of 5 × 107 cell/mL. For in vitro stimulation, fixed Candida preparations were made as previously described (21).
Murine model of oral candidiasis
C57BL/6 and DEFB1−/− mice were infected orally with C. albicans as previously detailed (21). Briefly, mice were pretreated with tetracycline containing drinking water for 5 d prior to infection. Mice were anesthetized using an Acepromazine/Ketamine cocktail and the dorsum of the tongues scratched using a sterile scalpel. After 4 h recovery, mice were orally infected with 5 × 106 yeast cells. At the time of sacrifice, a clinical score was assigned to quantify gross infection severity. Mucosal organs and kidneys were harvested, homogenized, plated on SD Agar plates and colonies counted after 48 h.
Tissue section preparation and histology
Serial cryo-sections (5 μm) of Candida infected wild-type (WT) and DEFB1−/− tongues were stained for DNA (DAPI) and neutrophils (NIMP R14, specific for murine Ly-6G/GR-1 and Ly-6C). Tongues sections were also stained with Periodic acid-Schiff with a hematoxylin counter-stain (PASH). Slides were then imaged on a Leica DMI 6000 B inverted microscope using a 40x objective attached to a Retiga EXI camera (Q-imaging). Image analysis was performed using MetaMorph Software (Molecular Devices). To quantify the levels of neutrophil infiltration, a percentage of NIMP+ epithelium was determined by dividing the number of NIMP+ pixels by the total number of pixels in a region of the epithelium.
Peritoneal neutrophil infiltration assay
C57BL/6, DEFB1−/−, and IL-1R1−/− mice were injected with 5 × 106 colony forming units (CFU) of C. albicans in 1 mL sterile PBS. After 16 h, the peritoneal cavity of mice was lavaged with 10 mL of DMEM media without FCS. Cells were centrifuged and counted on a hemacytometer. Diff-Quik stain (Fisher Scientific) was used to determine the portion of cells which were neutrophils.
XTT Assay for fungal killing
Peritoneal neutrophils were isolated by lavage following thioglycollate injection for 18 hours. Isolated cells were separated by Ficoll-Plaque by centrifuging lavaged cells at 500 × g for 30 minutes at 4°C. Resulting pellet was resuspended in RBC lysis buffer and following washing, neutrophil purity determined to be >90% by Cytospin/Diff Quick staining. 250,000 neutrophils were added to wells of a 96 well plate coated one hour prior with 250,000 Candida yeast in DMEM media. After 3 hour incubation at 37-C and 5% CO2, cells were pelleted at 1500 × g for 5 min. Media was aspirated off and neutrophils lysed by the addition of dH2O. Fungal viability is determined by XTT assay as detailed previously (22). Wells containing only Candida serve as controls and percent killing determined by (1-(exp. value/control value))*100.
Quantitative reverse transcriptase PCR
Buccal tissue was excised at 3 d post infection and stabilized in RNAlater (Qiagen). Tissue was homogenized and mRNA extracted using PrepEase RNA extraction kit (USB). cDNA was synthesized using high capacity cDNA synthesis kit (Applied Biosystems). Quantitative real-time PCR was performed on ABI 7500 qPCR machine (ABI) using Sybr Green Master Mix (Roche) with gene specific primers (Table S1). Expression was determined using the ΔΔCt method using the housekeeping gene GAPDH (23). NCBI gene accession numbers are as follows: Nlrc4: NM_001033367.3; Nlrp3: NM_145827.3; Il1b: NM_008361.3; Il17a: NM_010552.3; Il17f: NM_145856.2; Il18: NM_008360.1; CXCL1: NM_008176.3; Il6: NM_031168.1; Defb2:NM_010030.1; Defb3: NM_013756.2; Defb4: NM_019728.4; Defb14: NM_183026.2; Camp: NM_009921.2.
Cytokine assays
Circulating blood was collected from infected C57BL/6 and DEFB1−/− mice and serum was separated by centrifugation, and cytokine concentrations were determined by ELISA (R&D Systems). Supernatants were isolated from macrophages stimulated in vitro with 100 ng/mL of LPS for 4 hours, followed by 5 mM ATP for 30 minutes, or overnight stimulation with formalin-fixed germ tube C. albicans GDH2346 (previously identified to be the most immunostimulatory stage (21)), and cytokine production was measured by ELISA (R&D Systems).
Statistical analysis
Data were analyzed using commercial software (GraphPad Prism). Mann Whitney U-test was used for the statistical analysis of quantitative fungal burdens, qPCR, and ELISA data. P values are presented when statistical significance is observed (P ≤ 0.05 on a 95% confidence interval).
Results
mBD1 is required for control of oral Candida infection
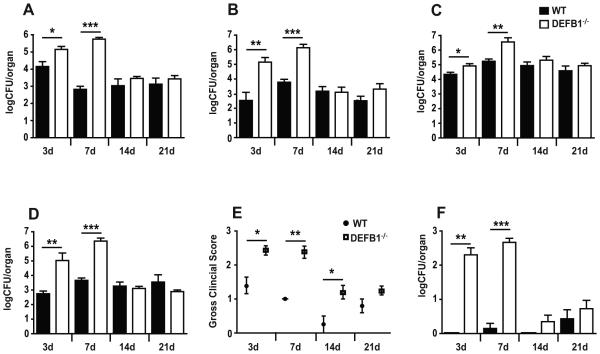
Release of antimicrobial peptides from mucosal tissues represents one of the first lines of host defense against pathogenic microorganisms. In order to assess the impact of mBD1 on control of oral Candida growth, we infected WT and DEFB1−/− mice using a previously published model of oropharyngeal candidiasis (21). We observed a significantly higher fungal growth in the oral cavity of DEFB1−/− compared to WT mice after 3 days of infection, and persisting to 7 days post-infection (Fig. 1A). Oral fungal loads were similar between WT and DEFB1−/− mice at 2 days post infection, demonstrating that the enhanced fungal colonization observed at later timepoints is not due to differences in initial infective loads (Supplemental Figure 1A). A concurrent increase in mucosal Candida infection was observed throughout the gastrointestinal tract including esophagus (Fig. 1B), stomach (Fig. 1C), and ileum (Fig. 1D). In addition to fungal burdens, a gross clinical score was assigned at each experimental endpoint to quantify the clinical severity of fungal infection by visual inspection of the oral and pharyngeal cavities. As expected, DEFB1−/− mice exhibited significantly elevated clinical disease in early infection (days 3, 7), which correlates with the increased fungal burdens at these endpoints (Fig. 1E). To determine the impact of mBD1 on prevention of systemic dissemination, we measured fungal burdens in the kidneys (a marker of blood stream dissemination of infection). DEFB1−/− mice showed significantly increased fungal burdens in the kidneys on days 3 and 7 compared to WT mice (Fig. 1F). In contrast to oral fungal loads, a trend towards increased fungal colonization of the kidneys was observed in DEFB1−/− mice at both 1 and 2 days post infection (Supplemental Figure 1B,C). By 14 days post infection, fungal colonization of tissues in the DEFB1−/− mice returned to WT levels, reinforcing the key role of antimicrobial peptides during early mucosal defense.
Figure 1. β-Defensin 1 protects against early mucosal infection and controls systemic dissemination with Candida albicans.
Quantitative fungal burdens of (A) Tongues, (B) Esophagus, (C) Stomach, and (D) Ileum of WT and DEFB1−/− mice after oral infection for 3, 7, 14 and 21 d with Candida. (E) Clinical severity of infection was determined by gross examination of tongues. (F) Systemic dissemination was determined by kidney fungal loads. All bars represent an N ≥ 5 mice pooled from a minimum of two experiments. (Data shown are means ± SEM; ***P < 0.001, ** P < 0.01, * P < 0.05)
Impaired neutrophil infiltration in Candida infected DEFB1−/− mice
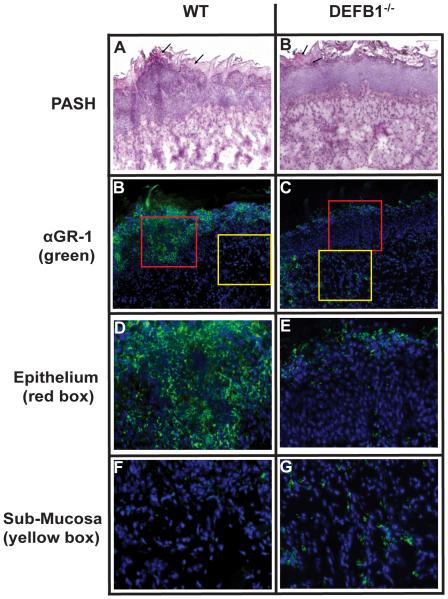
Given the pronounced impact of mBD1 deficiency on fungal clearance from mucosal organs, we sought to establish the etiology of this defective anti-fungal response. Prior reports have indicated that the extravasation and chemotaxis of neutrophils to sites of fungal infections is crucial for proper control and clearance of the pathogen. In order to assess neutrophil infiltration in our murine model of OPC, we isolated the tongues of WT and DEFB1−/− mice at 2 days post-infection, a timepoint chosen due to our finding that differences in oral fungal colonization manifests between days 2 and 3 post-infection. Sections were then stained with the NIMP R14 antibody, which recognizes Ly-6G/GR-1 and Ly-6C, cell surface proteins highly expressed on neutrophils and DAPI to identify nuclei. WT mice exhibited significant cellular infiltration in the epithelium surrounding fungal hyphae (Fig. 2A). These areas of dense cellularity corresponded with substantial levels of NIMP staining, identifying these cells as neutrophils (Fig. 2C). Although tissue erosion and visible hyphae were identified in the outer stratum corneum (para-keratinized stratified squamous epithelium on dorsum of tongue) of DEFB1−/− mice (Fig. 2B), the level of cellular infiltration observed in WT mice was noticeably greater (Fig. 2A). Further examination of the tongues revealed that the neutrophils appeared to substantially accumulate only in the stromal tissues in DEFB1−/− mice as compared to WT controls where neutrophils are seen within both the epithelium and stratum corneum (Fig. 2E-H). To quantify the level of neutrophil influx, we determined the percentage of tongue epithelium that stained positive for neutrophils. As can be seen in Supplemental Figure 2, there is nearly a 50% reduction in the percent of epithelium which stains positive for neutrophils in DEFB1−/− mice when compared to WT. Interestingly, when examining the tissue underlying the epithelium, there is minimal discernible difference in the presence of neutrophils indicating mBD1 deficiency may impact neutrophil chemotaxis to a more profound degree than neutrophil extravasation from the circulation.
Figure 2. Neutrophil infiltration in response to Candida requires mBD1.
Sections of Candida infected tongues from (A) WT and (B) DEFB1−/− mice were stained for fungal hyphae with PASH. Serial sections of (C) WT and (D) DEFB1−/− tongues were then stained with the neutrophil - specific NIMP antibody and nuclear stain DAPI. Magnified 40 × images of the epithelium (red squares; E, F) and sub-mucosa (yellow squares; G, H).
DEFB1−/− mice exhibit intact innate immune function
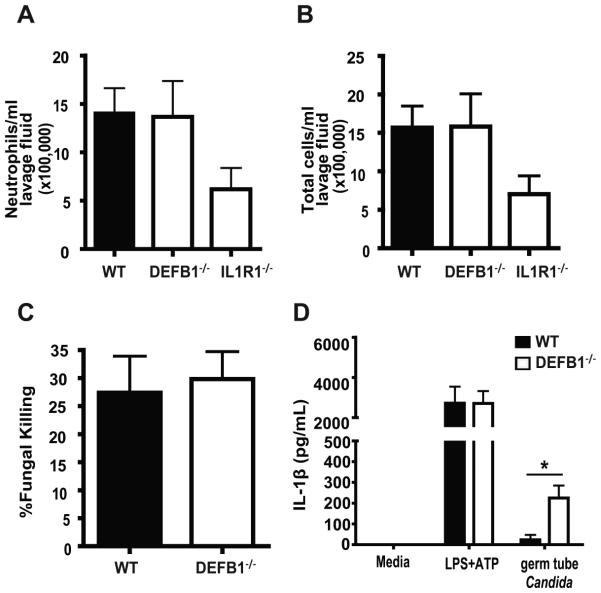
To help discern the role of mBD1 in neutrophil chemotaxis versus extravasations, the peritoneal cavities of WT and DEFB1−/− mice were injected with live Candida and neutrophil counts were obtained from lavage fluid 16 hours post-injection. Interestingly, we observed no difference in the levels of neutrophil accumulation between WT and DEFB1−/− mice (Fig. 3A). Total cellular infiltration was also equivalent between WT and DEFB1−/− mice (Fig. 3B). IL-1R1 −/− mice were used as a control for defective neutrophil extravasation, as we have previously shown the importance of IL-1 in host defense to Candida (21).
Figure 3. Intact innate immune function in DefB1−/− mice.
Peritoneal lavage of thioglycollate injected WT and mBD1 deficient hosts was used to determine levels of (A) Neutrophils and (B) Total cells. (C) The fungal killing capacity of purified WT and KO neutrophils was determined by XTT assay. (D) IL-1β release from macrophages was assessed by ELISA in response to LPS+ATP and germ tube Candida. Statistical significance determined by student t-test (* P < 0.05). No statistical difference was observed for neutrophil extravasation or killing.
Given the potential that the enhanced oral fungal colonization observed in the DEFB1−/− mice could be due to impaired neutrophil function, we compared fungal killing in vitro by WT and DEFB1−/− neutrophils utilizing a modified XTT assay. As seen in Figure 3C, there is no appreciable difference in the Candida killing capacity of neutrophil isolated from DEFB1−/− compared to WT mice. Macrophages from DEFB1−/− mice also exhibited similar IL-1β release in response LPS followed by ATP, a known inducer of IL-1β processing and release, and slightly elevated release in response to Candida (Figure 3D).
mBD1 regulates the expression of other anti-microbial peptides in mucosal candidiasis
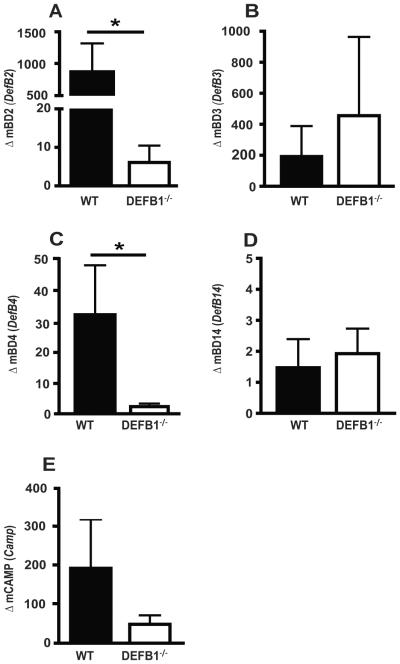
To assess the impact of mBD1 on inflammatory responses in the OPC infected mice, mRNA was extracted from buccal tissues of Candida infected mice at 3 days post infection and analyzed by quantitative PCR. One of the earliest innate immune responses to mucosal infection is the production of antimicrobial peptides. β-defensins have traditionally been thought to be constitutively expressed; however, some are induced following pathogenic infection. In our studies, WT mice exhibited elevated levels of mBD2, mBD3, and mBD4 following Candida infection (Fig. 4A-C). Expression levels of mBD2 and mBD4 were significantly reduced in Candida infected DEFB1−/− mice when compared to WT mice (Fig. 4A,C). Levels of mBD3 and mBD14 were similar in WT and DEFB1−/− mice (Fig. 4B,D). Cathelicidin/LL-37, or CAMP, is another antimicrobial peptide known to be important for protection from mucosal infections (24-26). Our previous work demonstrated a significant increase in CAMP expression following Candida infection (27). Here we show that CAMP levels are elevated in WT mice following infection but reduced in DEFB1−/− mice (Fig. 4E). To rule out the possibility that antibiotic treatment could alter innate immune activation in response to mucosal injury or infection, we measured the mRNA expression levels of key innate immune mediators in untreated naïve or tetracycline treated mice that were scored orally with a scalpel as described, then mock infected with PBS. None of the β-defensins or cytokines tested exhibited substantial alteration in tetracycline treated WT mucosa compared to naïve (no-tet) (Supplemental Figure 3).
Figure 4. mBD1 is critical for the upregulation of other antimicrobial peptides following oral Candida infection.
Expression of (A) β-Defensin 2, (B) β-Defensin 3, (C) β-Defensin 4, (D) β-Defensin 14, and (E) CAMP were determined in buccal mucosal tissues of WT and DEFB1−/− mice 3d post infection. All genes are normalized to GAPDH and graphed as fold induction over mock infection. Bars represent an N = 5 for WT mice and N = 7 for DEFB1−/− mice. (* P < 0.05)
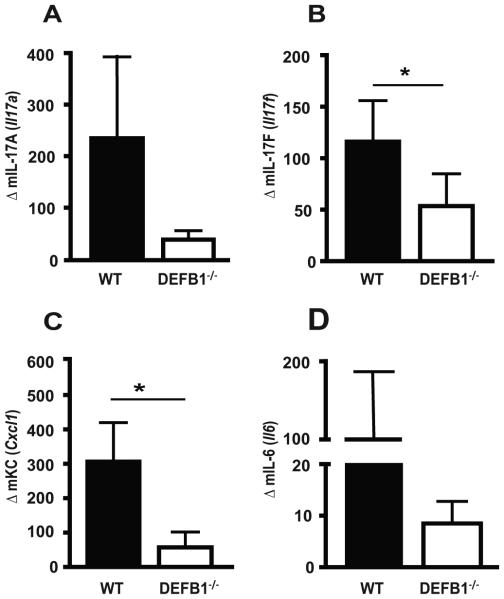
mBD1 is required for oral inflammatory responses to Candida, including IL-17, KC and IL-6
Given the established role for pro-inflammatory cytokines on the generation of protective responses to Candida, we next assessed the impact of mBD1 on cytokine expression. We first examined members of the IL-17 family, which are known to be important for the prevention and control of mucosal Candida infections (28-31). IL-17A levels were highly upregulated in WT mice following Candida infection, with a reduction observed in DEFB1−/− mice (Fig. 5A). IL-17F was also upregulated after introduction of Candida, with significantly reduced levels observed in DEFB1−/− (Fig. 5B). The chemokine KC is a known mediator of neutrophil infiltration into tissue. Expression of KC following Candida infection was reduced in DEFB1−/− mice in comparison to WT mice which exhibited a robust KC response (Fig. 5C). IL-6 had a similar expression pattern as IL-17A and KC (Fig. 5D).
Figure 5. Inflammatory cytokine expression following Candida infection is impaired in the absence of mBD1.
Buccal mRNA expression of (A) IL-17A, (B) IL-17F, (C) KC, and (D) IL-6 was determined 3 d after infection. All genes were normalized to GAPDH. Bars represent an N = 5 for WT mice, and N = 7 for DEFB1−/− mice. (* P < 0.05)
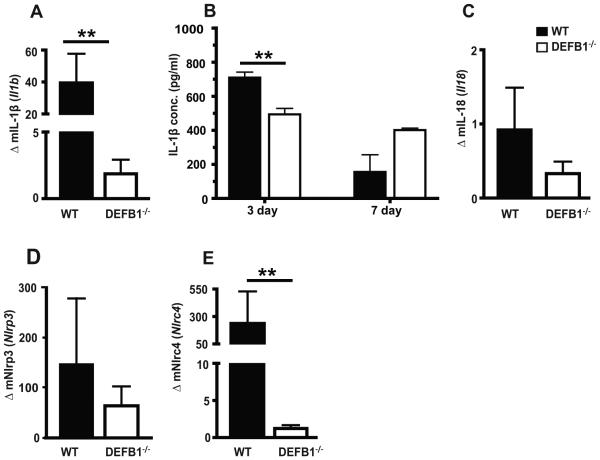
IL-1β responses to Candida infection is attenuated in DEFB1−/− mice
Our previous work has focused on the role of IL-1β and inflammasome activation on control of Candida infection (21, 27). A significant reduction in IL-1β expression was observed in DEFB1−/− mice when compared to WT mice (Fig. 6A). Serum levels of IL-1β were also reduced at 3 days in DEFB1−/− mice (Fig. 6B). IL-18 is another cytokine requiring inflammasome mediated activation. In our model, we did not observe induction of IL-18 in any of the mice after infection (Fig. 6C). Given the critical role for inflammasome expression and activation in host defense during Candida infection (21, 27, 32-34), inflammasome gene regulation was assessed. A robust upregulation of NLRP3 was observed in WT mice, with a non-significant trend towards lower expression in DEFB1−/− mice (Fig. 6D). In contrast, the induction of NLRC4, which we have previously shown to be important in mucosal responses to Candida, was significantly reduced in DEFB1−/− compared to WT mice (Fig. 6E).
Figure 6. Increased IL-1β and NLR expression after Candida infection is dependent on mBD1.
(A) IL-1β, (C) IL-18, (D) NLRP3, and (E) NLRC4 expression in the buccal mucosa was determined 3d post infection. (B) Circulating levels of IL-1β determined by ELISA from serum of Candida infected mice. Bars represent an N = 5 for WT mice, and N = 7 for DEFB1−/− for qPCR and serum represent pooled samples (two pools of 4 mice each). (** P < 0.01)
Discussion
The significant economic costs of fungal infections and emergence of drug resistant strains makes research into the precise mechanisms by which host immune systems combat fungal pathogens essential (35-38). The majority of current fungal immunology research focuses on the mechanisms by which host immune cells recognize fungus and how downstream production of cytokines induces a state of protective immunity. Despite numerous reports on the critical nature of β-defensins in response to pathogenic infections, there has been limited work on the role of these antimicrobial peptides in response to Candida, especially in mice. Using our non-immunosuppressed mouse model of oral Candida infection, we clearly demonstrate a novel role for mBD1 in immune-mediated protection from mucosal candidiasis. Mice deficient in this protein exhibited a nearly 10 fold higher level of oral infection at early time points (up to 7 days). By 14 days after infection, DEFB1−/− mice show similar levels of oral fungal burdens as WT mice, at which point it is likely adaptive immunity is able to compensate for the absence of mBD1. Gross inspection of the tongues of infected DEFB1−/− mice showed the presence of large confluent patches of fungus, a hallmark of thrush, during the first week of infection whereas WT mice had small isolated patches of fungus. This important new role for mBD1 in mucosal Candida infection is confirmed by the early and sustained increase in fungal burdens in other mucosal tissues including the esophagus, stomach, and ileum. Similar levels of oral infection were observed at 2 days post infection, identifying the time between 2 and 3 days post-infection as critical for mBD1 enhanced immunity.
Although mucosal Candida infections are common and constitute a major detriment to the quality of life of infected patients, bloodborne dissemination of Candida poses the greatest threat to public health due to the rapid mortality associated with this infection. Reports by our lab and others have previously shown that activation of innate immune responses, including inflammasome activation, in hematopoietic cells was critical for the prevention/control of bloodstream infections. Surprisingly, our current studies show that DEFB1−/− mice had significantly elevated levels of disseminated bloodstream infection at Days 3 and 7. Therefore, we show that mBD1 is not only responsible for limiting infection on mucosal surfaces but also for early prevention of fungal movement through infected tissues into blood vessels. Unlike the oral fungal infection levels which remained the same initially (up to Day 2), elevated fungal burdens were observed in the kidneys of DEFB1−/− mice at both Days 1 and 2 post infection. The nature of this early defect is unclear, but potential mechanisms could include altered mucosal barrier integrity in the gut or oral cavity, altered gut microflora and/or impaired systemic cellular immunity to blood stream fungal dissemination.
In order to detail the inflammatory response in DEFB1−/− mice, quantitative gene expression analysis was performed on infected tissue. A drastic increase in the expression of mBD2, mBD3, and mBD4 was seen following Candida infection. This is consistent with prior reports which observed increases in mBD3 and mBD4 expression in the tongue and stomach of Candida infected mice (39, 40). It was recently discovered that mBD3 and mBD4 upregulation requires the high mobility group nucleosomal binding domain 2 protein (HMGN2), a chromatin binding protein; however, mBD1 expression was shown to be independent of HMGN2, indicating that basal mBD1 expression may be uniquely regulated and a critical component of early mucosal responses (41). We observed a significant reduction in mBD2 and mBD4 expression in DEFB1−/− mice when compared to WT, demonstrating a possible cross-regulation of these peptides. Another antimicrobial protein, CAMP, was also upregulated in WT mice, with a drastic reduction observed in DEFB1−/− mice. This increase in CAMP is consistent with a previous study using a scarified corneal keratitis model (42). Recently, it has been demonstrated that LL-37, the human ortholog of CAMP, reduced Candida adhesion and viability (43), supporting an important role for this peptide in mucosal defense.
Human defensins have been hypothesized to be inducers of inflammatory responses in addition to known roles in chemotaxis (44-46). The induction of pro-inflammatory genes early after infection is a necessary component of immune responses to pathogenic organisms. We found that expression levels of both IL-17A and IL-17F were reduced in DEFB1−/− mice following Candida infection. Patients suffering from Hyper-IgE syndrome, a genetic defect in the STAT3 signaling pathway downstream of the IL-17R, are highly susceptible to mucosal Candida infection. It was recently found that these patients show decreased antimicrobial peptide production, including hBD2 (47). Thus, the reduction mBD2 expression that we observed in Candida infected DEFB1−/− mice may be due to the lower levels of IL-17 seen in these mice, showing the possible downstream effects of this important cytokine. The neutrophil chemokine KC (a homolog of human IL-8) was also found to be highly upregulated in WT mice, consistent with a prior report using a model of gastric candidiasis (40). KC is a potent chemokine, and plays a role in the recruitment of inflammatory cells such as neutrophils to sites of infection (48).
Given our previous work delineating the indispensable role for IL-1β in the clearance of Candida, we sought to determine the impact of mBD1 on IL-1 specific responses. The serum level of IL-1β expression was significantly reduced in DEFB1−/− mice following Candida infection, which may explain the persistently elevated infection levels observed at early timepoints. IL-1β release is a two-step process which requires not only the upregulation of pro-IL-1β but also proteolytic cleavage of the protein which is mediated by protein complexes termed inflammasomes (49). We have previously shown a critical role for the inflammasome molecule NLRP3 in host defense to both mucosal and disseminated Candida infection (21, 27). Additionally, we have shown a mucosal specific role for the NLRC4 inflammasome in control of mucosal candidiasis (27). Therefore, we investigated the impact of mBD1 deficiency on the induction of inflammasome genes in epithelial tissues of OPC infected mice. Interestingly, DEFB1−/− mice showed similar induction of NLRP3 after Candida infection as WT mice. However, NLRC4 expression was greatly reduced in DEFB1−/− mice, illustrating another potential mechanism of action. To our knowledge, this report is the first to link the production of an antimicrobial peptide with the transcriptional regulation of an inflammasome molecule.
We also show, by semi-quantitative analysis of tissue sections, a reduction of neutrophil infiltration into infected epithelium of DEFB1−/− mice. Neutrophils are known to have potent antifungal activity and are critical for control of infection. The substantial reduction in KC expression in DEFB1−/− mice may account for the defect observed in neutrophil recruitment to the areas of Candida mucosal infection, as this molecule has strong chemotactic properties (48), although other antimicrobial peptides may also play a role in cellular recruitment. Neutrophils in DEFB1−/− mice were observed to extravasate into the stromal tissues of the tongue at similar levels to WT mice but failed to infiltrate into the epithelium. The level of neutrophil infiltration in response to Candida in a peritoneal model was similar between WT and DEFB1 KO mice, showing that the neutrophils in DEFB1−/− mice do not have intrinsic defects in extravasation. Additionally, our in vitro studies show that the innate immune functioning and fungal killing of immune cells isolated from the DEFB1−/− mice was intact. Taken together, these studies identify defective trafficking of neutrophils to the mucosal location of Candida infection as a crucial component of the increased susceptibility to oral candidiasis that results from deficiency in mBD1.
One explanation for this phenomenon may be the capacity for β-defensins to bind to chemokine receptors. Recent reports have found that hBD1 and hBD2 were capable of binding to and activating CCR6 (14, 50). One of these reports also identified MIP3α, the canonical chemokine for CCR6, as possessing antimicrobial activity (50). A more recent report found that MIP3a had anti-Candida activity (51). Similar to these studies, CXCL9, CXCL10, and CXCL11 have also been identified as having antimicrobial activities (52). These point to an evolutionary relationship between β-defensins and chemokines, suggesting defensins not only function as antimicrobial peptides but that they can signal through chemokine receptors to directly mediate leukocyte trafficking.
This report identifies a novel role for mBD1 in host defense to mucosal fungal infections. We hypothesize that the role of mBD1 in protection from mucosal candidiasis is occurring through one or more different mechanisms of action; direct antimicrobial activity, regulation of host inflammatory responses, including the augmentation of cytokine and AMP responses, and mediating chemotactic attraction (directly or via a second signal) of inflammatory cells such as neutrophils to the sites of mucosal infection. The emergence of drug-resistance Candida sp. has spurred research into the development of novel anti-fungal therapies. This report has identified β-defensin 1 as a potentially novel target for therapeutic development.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of S. Howell (imaging assistance), B. Reuter and T. Pizarro for providing DEFB1−/− mice, B. Reuter for genotyping analysis, and H. Butler and K. Zongolowicz for animal husbandry and genotyping assistance.
Footnotes
Corresponding Author: Amy G. Hise, MD, MPH, Department of Pathology, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106-4983, Tel: 216 368 5036, Fax: 216 368 0494, amy.hise@case.edu
- AMP
- antimicrobial peptide
- mBD
- murine β-defensin
- hBD
- human β-defensin
- OPC
- oropharyngeal candidiasis
- qPCR
- quantitative real-time PCR
- WT
- wild type
This work was supported by NIH grant R01DE018279 (AGH), T32GM008056 (JT). Histology and microscopy services were supported by P30EY11373.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan WC. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15(3):414–21. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 3.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469(7330):419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Jiang Y, Gong T, Cui X, Li W, Feng Y, Wang B, Jiang Z, Li M. High-level expression and novel antifungal activity of mouse beta defensin-1 mature peptide in Escherichia coli. Appl Biochem Biotechnol. 2010;160(1):213–21. doi: 10.1007/s12010-009-8566-3. [DOI] [PubMed] [Google Scholar]
- 6.Hassan SA. Amino acid side chain interactions in the presence of salts. J Phys Chem B. 2005;109(46):21989–96. doi: 10.1021/jp054042r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vylkova S, Li XS, Berner JC, Edgerton M. Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother. 2006;50(1):324–31. doi: 10.1128/AAC.50.1.324-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vylkova S, Nayyar N, Li W, Edgerton M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother. 2007;51(1):154–61. doi: 10.1128/AAC.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scudiero O, Galdiero S, Cantisani M, Di Noto R, Vitiello M, Galdiero M, Naclerio G, Cassiman JJ, Pedone C, Castaldo G, Salvatore F. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Chemother. 2010;54(6):2312–22. doi: 10.1128/AAC.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JK, Park YJ, Kum KY, Han SH, Chang SW, Kaufman B, Jiang J, Zhu Q, Safavi K, Spangberg L. Antimicrobial efficacy of a human beta-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int Endod J. 2012 doi: 10.1111/iej.12002. [DOI] [PubMed] [Google Scholar]
- 11.Argimon S, Fanning S, Blankenship JR, Mitchell AP. Interaction between the Candida albicans high-osmolarity glycerol (HOG) pathway and the response to human beta-defensins 2 and 3. Eukaryot Cell. 2011;10(2):272–5. doi: 10.1128/EC.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bals R, Goldman MJ, Wilson JM. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66(3):1225–32. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinrichsen K, Podschun R, Schubert S, Schroder JM, Harder J, Proksch E. Mouse beta-defensin-14, an antimicrobial ortholog of human beta-defensin-3. Antimicrob Agents Chemother. 2008;52(5):1876–9. doi: 10.1128/AAC.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and Biological Characterization of Mouse beta-defensin 14, the orthologue of human beta-defensin 3. J Biol Chem. 2008;283(9):5414–9. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 15.Jia HP, Wowk SA, Schutte BC, Lee SK, Vivado A, Tack BF, Bevins CL, McCray PB., Jr A novel murine beta -defensin expressed in tongue, esophagus, and trachea. J Biol Chem. 2000;275(43):33314–20. doi: 10.1074/jbc.M006603200. [DOI] [PubMed] [Google Scholar]
- 16.Moser C, Weiner DJ, Lysenko E, Bals R, Weiser JN, Wilson JM. beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70(6):3068–72. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanida T, Okamoto T, Okamoto A, Wang H, Hamada T, Ueta E, Osaki T. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J Oral Pathol Med. 2003;32(10):586–94. doi: 10.1034/j.1600-0714.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 18.Ryan LK, Freeman KB, Masso-Silva JA, Falkovsky K, Aloyouny A, Markowitz K, Hise AG, Fatahzadeh M, Scott RW, Diamond G. Activity of potent and selective host defense peptide mimetics in mouse models of oral candidiasis. Antimicrob Agents Chemother. 2014;58(7):3820–7. doi: 10.1128/AAC.02649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Yi X, Li M, Wang T, Qi T, She X. Antimicrobial activities of recombinant mouse beta-defensin 3 and its synergy with antibiotics. J Mater Sci Mater Med. 2012;23(7):1723–8. doi: 10.1007/s10856-012-4645-z. [DOI] [PubMed] [Google Scholar]
- 20.Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009;53(1):256–60. doi: 10.1128/AAC.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5(5):487–97. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, Dongari-Bagtzoglou A. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One. 2011;6(1):e16218. doi: 10.1371/journal.pone.0016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. Journal of Immunology. 2004;172(3):1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125(1):108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 26.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95(16):9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7(12):e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 30.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, Netea MG. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5(4):329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37(10):2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 32.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–6. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 33.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183(6):3578–81. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183(12):8061–7. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 35.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010;28(2):197–201. doi: 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, - Diagn Microbiol Infect Dis. 2012;74(4):323–31. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125(1 Suppl):S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Schofield DA, Westwater C, Balish E. beta-defensin expression in immunocompetent and immunodeficient germ-free and Candida albicans-monoassociated mice. J Infect Dis. 2004;190(7):1327–34. doi: 10.1086/423856. [DOI] [PubMed] [Google Scholar]
- 40.Schofield DA, Westwater C, Balish E. Divergent chemokine, cytokine and beta-defensin responses to gastric candidiasis in immunocompetent C57BL/6 and BALB/c mice. J Med Microbiol. 2005;54:87–92. doi: 10.1099/jmm.0.45755-0. Pt 1. [DOI] [PubMed] [Google Scholar]
- 41.Deng LX, Wu GX, Cao Y, Fan B, Gao X, Tang XH, Huang N. The chromosomal protein HMGN2 mediates the LPS-induced expression of beta-defensins in mice. Inflammation. 2012;35(2):456–73. doi: 10.1007/s10753-011-9335-3. [DOI] [PubMed] [Google Scholar]
- 42.Yuan X, Hua X, Wilhelmus KR. The corneal expression of antimicrobial peptides during experimental fungal keratitis. Curr Eye Res. 2010;35(10):872–9. doi: 10.3109/02713683.2010.495812. [DOI] [PubMed] [Google Scholar]
- 43.Chang HT, Tsai PW, Huang HH, Liu YS, Chien TS, Lan CY. LL37 and hBD-3 elevate the beta-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem J. 2012;441(3):963–70. doi: 10.1042/BJ20111454. [DOI] [PubMed] [Google Scholar]
- 44.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, Kajiwara N, Saito H, Nagaoka I, Ogawa H, Okumura K. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184(7):3526–34. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 45.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175(3):1776–84. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 46.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127(3):594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 47.Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4(4):448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–7. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 49.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 50.Hoover DM, Boulegue C, Yang D, Oppenheim JJ, Tucker K, Lu W, Lubkowski J. The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem. 2002;277(40):37647–54. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 51.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74(3):448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 52.Cole AM, Ganz T. Human antimicrobial peptides: analysis and application. Nature Medicine. 2001;7(2):158–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.