Abstract
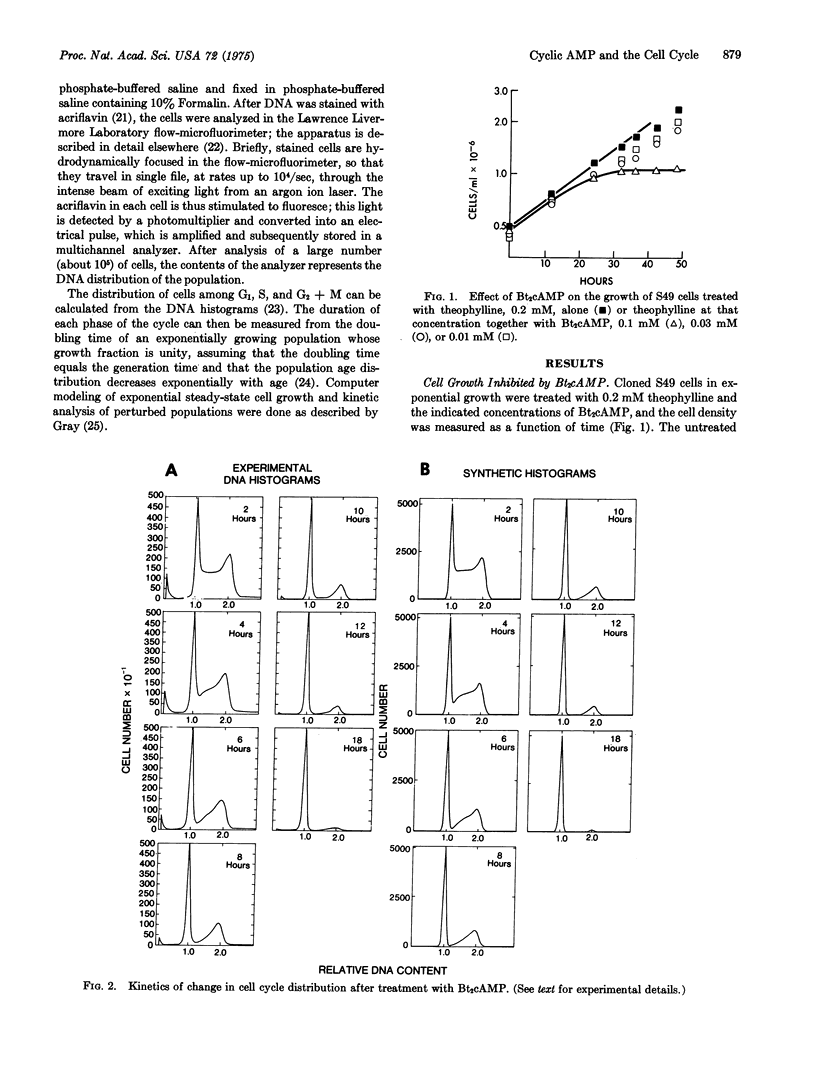
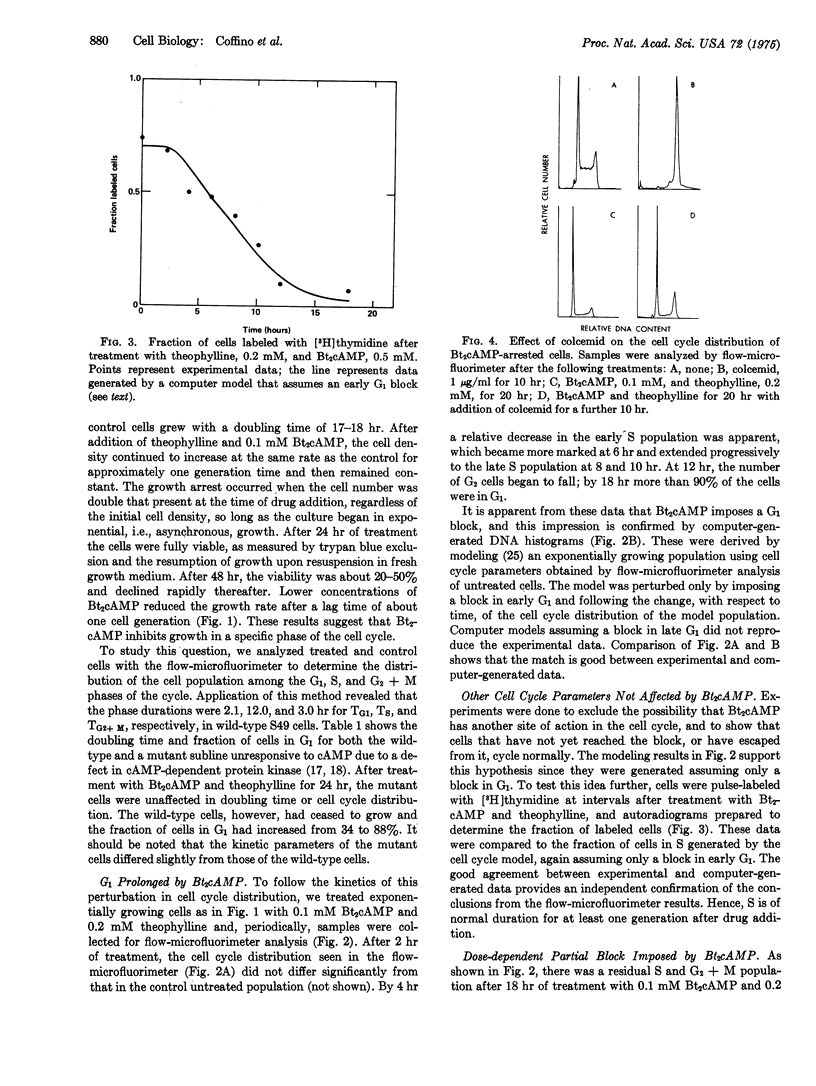
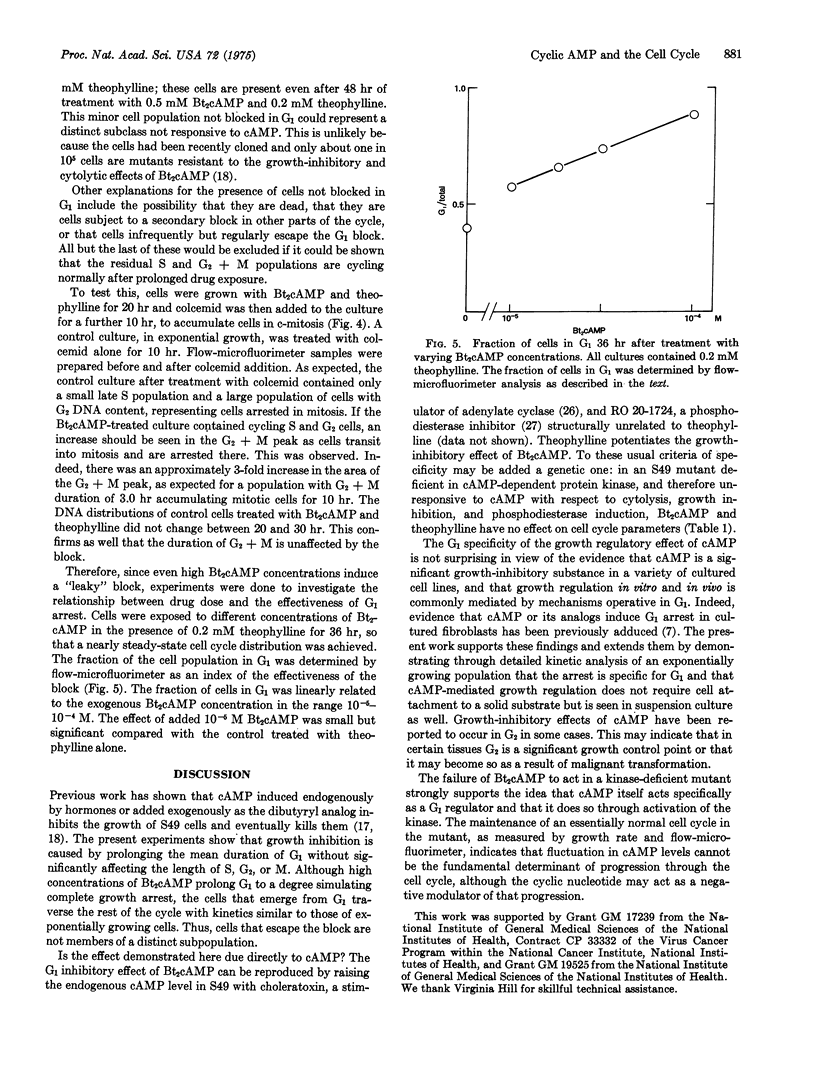
Flow-microfluorimetric analysis has been carried out on populations of exponentially growing S49 mouse lymphoma cells treated with dibutyryl cyclic AMP. The drug produces a specific concentration-dependent block in the G-1 phase of the cell cycle while other phases of the cycle are not perceptibly altered. The cell cycle of a line of mutant cells lacking the cyclic AMP-dependent protein kinase is not affected by the drug. Since these mutant cells have been shown to maintain a normal cell cycle, even in the presence of high levels of cyclic AMP, periodic fluctuations in the levels of the cyclic nucleotide cannot be required for or determine progression through the cell cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Russell T. R., Carchman R. A., Pastan I. Interrelationship between adenylate cyclase activity, adenosine 3':5' cyclic monophosphate phosphodiesterase activity, adenosine 3':5' cyclic monophosphate levels, and growth of cells in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3802–3805. doi: 10.1073/pnas.70.12.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. K., Zlotnick B. J. Growth-and density-dependent inhibition of deoxyglucose transport in Balb 3T3 cells and its absence in cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2374–2378. doi: 10.1073/pnas.70.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Daniel V., Litwack G., Tomkins G. M. Induction of cytolysis of cultured lymphoma cells by adenosine 3':5'-cyclic monophosphate and the isolation of resistant variants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):76–79. doi: 10.1073/pnas.70.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W. Cyclic 3':5' AMP and cell proliferation in cultures of embryonic rat cells. Exp Cell Res. 1972 Mar;71(1):238–241. doi: 10.1016/0014-4827(72)90287-x. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. W. Cell cycle analysis from computer synthesis of deoxyribonucleic acid histograms. J Histochem Cytochem. 1974 Jul;22(7):642–650. doi: 10.1177/22.7.642. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Schroeder J. L. Dibutyryl cyclic adenosine 3'5' monophosphate, sugar transport, and regulatory control of cell division in normal and transformed cells. J Cell Biol. 1973 Feb;56(2):487–491. doi: 10.1083/jcb.56.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Mak S. Mammalian cell cycle analysis using microspectrophotometry combined with autoradiography. Exp Cell Res. 1965 Aug;39(1):286–289. doi: 10.1016/0014-4827(65)90030-3. [DOI] [PubMed] [Google Scholar]
- Oey J., Vogel A., Pollack R. Intracellular cyclic AMP concentration responds specifically to growth regulation by serum. Proc Natl Acad Sci U S A. 1974 Mar;71(3):694–698. doi: 10.1073/pnas.71.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Remington J. A., Klevecz R. R. Hormone-treated CHO cells exit the cell cycle in the G2 phase. Biochem Biophys Res Commun. 1973 Jan 4;50(1):140–146. doi: 10.1016/0006-291x(73)91075-9. [DOI] [PubMed] [Google Scholar]
- Sheppard H., Wiggan G. Different sensitivities of the phosphodiesterases (adenosine-3',5'-cyclic phosphate 3'-phosphohydrolase) of dog cerebral cortex and erythrocytes to inhibition by synthetic agents and cold. Biochem Pharmacol. 1971 Aug;20(8):2128–2130. doi: 10.1016/0006-2952(71)90426-6. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R., Prescott D. M. Cyclic AMP levels in synchronized mammalian cells. Exp Cell Res. 1972 Nov;75(1):293–296. doi: 10.1016/0014-4827(72)90554-x. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets L. A. Contact inhibition of transformed cells incompletely restored by dibutyryl cyclic AMP. Nat New Biol. 1972 Sep 27;239(91):123–124. doi: 10.1038/newbio239123a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Medley G., Lingwood C. A. Growth inhibition of murine tumor cells, in vitro, by puromycin, ( 6 N)O 2 '-dibutyryl 3',5'-adenosine monophosphate, or adenosine: evidence of commitment for cell division. J Cell Biol. 1973 May;57(2):397–405. doi: 10.1083/jcb.57.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., Coulter J. R. Cell microfluorometry: a method for rapid fluorescence measurement. Science. 1969 Mar 14;163(3872):1213–1214. doi: 10.1126/science.163.3872.1213. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Johnson G. S., Pastan I. Control of DNA synthesis and mitosis in 3T3 cells by cyclic AMP. Biochem Biophys Res Commun. 1972 Aug 21;48(4):743–748. doi: 10.1016/0006-291x(72)90669-9. [DOI] [PubMed] [Google Scholar]


