Abstract
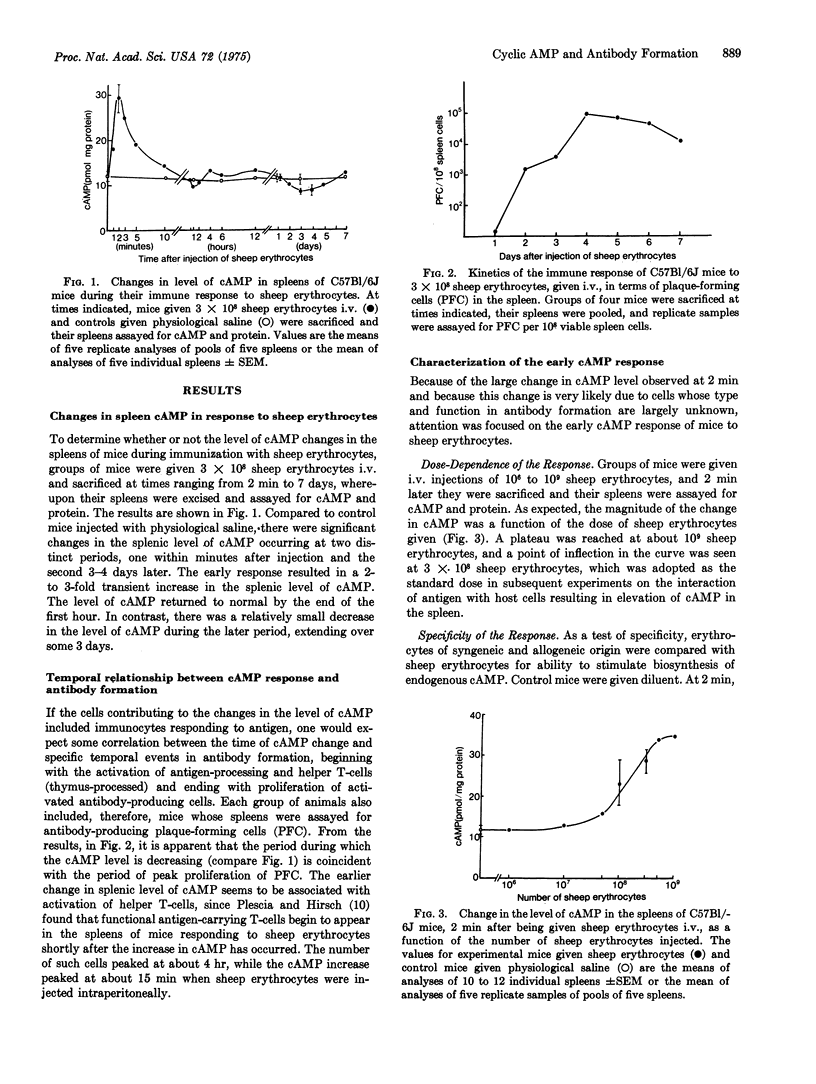
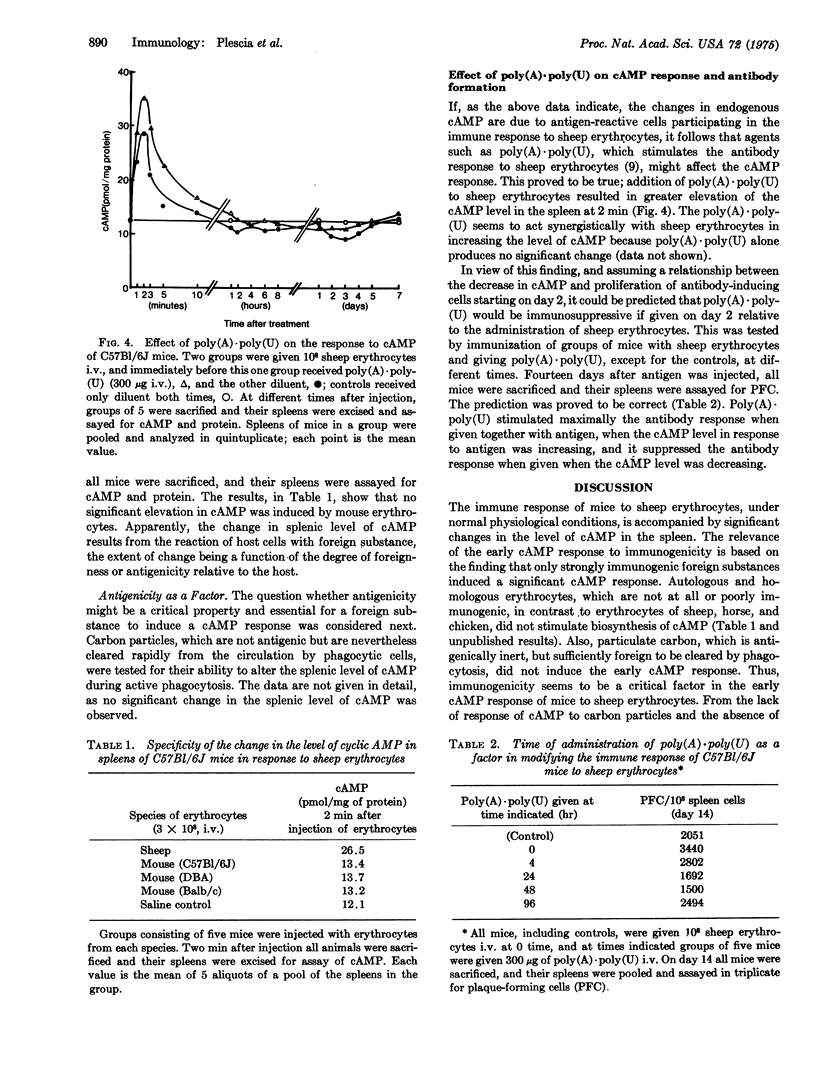
Intravenous injection of sheep erythrocytes into normal immunologically competent C57BL/6J mice results in significant and characteristic changes in the splenic level of 3':5'-cAMP with initiation of the immune response and proliferation of antibody-forming cells. The level increases 2- to 3-fold initially, peaks at 2 min, and returns to base level in an hour. Between 2 and 5 days there is a decrease, followed by a peak when the rate of proliferation of antibody-forming cells is maximal. Changes in splenic level of cAMP are thus transitory and biphasic, and they occur only in response to foreign substances that are immunogenic, such as heterologous erythrocytes, and not to antigenically inert carbon particles. They are also dependent upon the dose of immunogen. Moreover, the double-stranded hybrid of polyadenylate and polyuridylate, which acts synergistically with antigen in stimulating endogenous cAMP, is immuno-enhancing if given with sheep erythrocytes when the cAMP level is increasing, and immunosuppresive if given when cAMP is decreasing. These data provide direct evidence for a role of cAMP as a mediator in the activation and proliferation of immunocytes stimulated by antigen. With knowledge of the transitory and biphasic nature of the cAMP response induced by antigen, one can avoid indiscriminate use of drugs that modify the level of endogenous cAMP and instead employ them rationally in controlling the immune response, enhancing or suppressing it as desired.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Kon C. An improved protein binding assay for cyclic AMP. Anal Biochem. 1974 Apr;58(2):459–468. doi: 10.1016/0003-2697(74)90214-0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Plescia O. J., Hursch J. S. Discussion paper: isolation and characterization of functional antigen-carrying T cells. Ann N Y Acad Sci. 1973 May 31;207:49–56. doi: 10.1111/j.1749-6632.1973.tb47475.x. [DOI] [PubMed] [Google Scholar]
- Schell-Frederick E., Van Sande J. Evidence that cyclic AMP is not the chemical mediator of the metabolic changes accompanying phagocytosis. J Reticuloendothel Soc. 1974 Feb;15(2):139–148. [PubMed] [Google Scholar]
- Teh H. S., Paetkau V. Biphasic effect of cyclic AMP on an immune response. Nature. 1974 Aug 9;250(5466):505–507. doi: 10.1038/250505a0. [DOI] [PubMed] [Google Scholar]


