Abstract
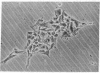
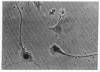
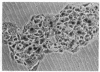
Melanocyte stimulating hormone (MSH) enhances melanization but inhibits proliferation of Cloudman S91 melanoma cells in culture. We have isolated variants of these cells that can grow in the presence of MSH. The conclusions we have reached from analyses of these cells are the following: (1) Basal tyrosinase activity (monophenol monooxygenase; monophenol, dihydroxyphenylalanine:oxygen oxidoreductase, EC 1.14.18.1), i.e., the activity that is present in the absence of added MSH, is related through a common biochemical pathway to MSH-mediated control of growth. (2) MSH-inducible tyrosinase activity does not appear to be related to MSH control of growth. (3) The morphological changes that occur following the addition of MSH or cAMP are related to controls of growth and not to those of melanization.
Full text
PDF
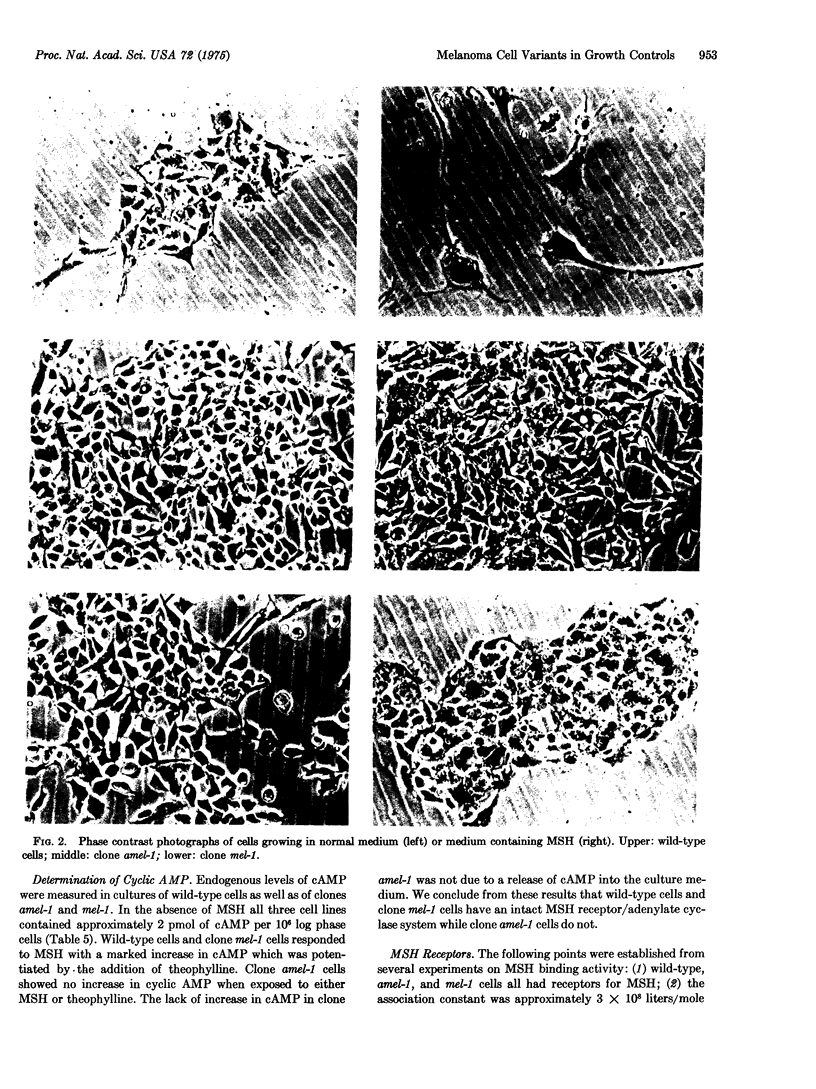
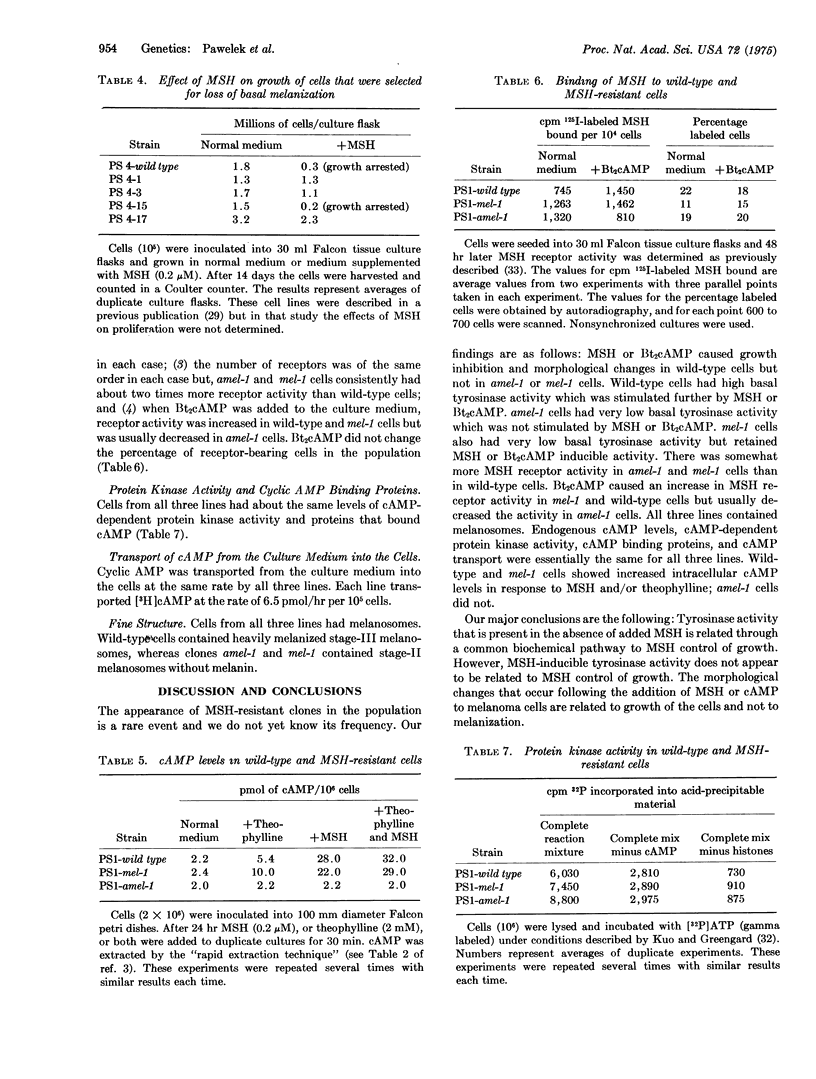

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S., Sheppard J. R. Cyclic AMP, ATP and cell contact. Nature. 1974 Jul 5;250(461):62–64. doi: 10.1038/250062a0. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Gullino P. M. In vivo inhibition of growth of two hormone-dependent mammary tumors by dibutyryl cyclic AMP. Science. 1974 Jan 11;183(4120):87–88. doi: 10.1126/science.183.4120.87. [DOI] [PubMed] [Google Scholar]
- Daniel V., Litwack G., Tomkins G. M. Induction of cytolysis of cultured lymphoma cells by adenosine 3':5'-cyclic monophosphate and the isolation of resistant variants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):76–79. doi: 10.1073/pnas.70.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J., Schroeder J. L. Dibutyryl cyclic adenosine 3'5' monophosphate, sugar transport, and regulatory control of cell division in normal and transformed cells. J Cell Biol. 1973 Feb;56(2):487–491. doi: 10.1083/jcb.56.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Cyclic AMP-treated sarcoma cells acquire several morphological characteristics of normal fibroblasts. Ann N Y Acad Sci. 1971 Dec 30;185:413–416. doi: 10.1111/j.1749-6632.1971.tb45267.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Role of 3',5'-adenosine monophosphate in regulation of morphology and growth of transformed and normal fibroblasts. J Natl Cancer Inst. 1972 May;48(5):1377–1387. [PubMed] [Google Scholar]
- Keller R., Keist R. Suppression of growth of P-815 mastocytoma cells in vitro by drugs increasing cellular cyclic 3',5'-adenosine monophosphate. Life Sci II. 1973 Feb 8;12(3):97–105. doi: 10.1016/0024-3205(73)90326-3. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Lee M. S., Lu M. Y. Effects of alpha-MSH on melanogenesis and tyrosinase of B-16 melanoma. Endocrinology. 1972 Nov;91(5):1180–1188. doi: 10.1210/endo-91-5-1180. [DOI] [PubMed] [Google Scholar]
- Lim R., Mitsunobu K. Effect of dibutyryl cyclic AMP on nucleic acid and protein synthesis in neuronal and glial tumor cells. Life Sci II. 1972 Nov 8;11(21):1063–1070. doi: 10.1016/0024-3205(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis A. J., Forrest G., Pious D. A. Cyclic AMP in cultured human lymphoid cells: relationship to mitosis. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1645–1649. doi: 10.1016/0006-291x(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Morley A., Quesenberry P., Garrity M., Stohlman F., Jr Inhibition of marrow growth by cyclic AMP. Proc Soc Exp Biol Med. 1971 Oct;138(1):57–59. doi: 10.3181/00379727-138-35831. [DOI] [PubMed] [Google Scholar]
- Newsome D. A., Fletcher R. T., Robison W. G., Jr, Kenyon K. R., Chader G. J. Effects of cyclic AMP and Sephadex fractions of chick embryo extract on cloned retinal pigmented epithelium in tissue culture. J Cell Biol. 1974 May;61(2):369–382. doi: 10.1083/jcb.61.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J., Sansone M., Morowitz J., Moellmann G., Godawska E. Genetic control of melanization: isolation and analysis of amelanotic variants from cultured melanotic melanoma cells. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1073–1077. doi: 10.1073/pnas.71.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J., Wong G., Sansone M., Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J Biol Med. 1973 Dec;46(5):430–443. [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H. L-tyrosine-3,5-3H assay for tyrosinase development in skin of newborn hamsters. Science. 1969 May 16;164(3881):838–839. doi: 10.1126/science.164.3881.838. [DOI] [PubMed] [Google Scholar]
- Rein A., Carchman R. A., Johnson G. S., Pastan I. Simian virus 40 rapidly lowers cAMP levels in mouse cells. Biochem Biophys Res Commun. 1973 Jun 8;52(3):899–904. doi: 10.1016/0006-291x(73)91022-x. [DOI] [PubMed] [Google Scholar]
- Sandor R. Inhibition of human rhabdomyosarcoma-cell growth in agar by dibutyryl cyclic AMP. J Natl Cancer Inst. 1973 Jul;51(1):257–260. doi: 10.1093/jnci/51.1.257. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel R. W., Hall R. G. Effect of dibutyryl cyclic AMP on the restoration of contact inhibition in tumor cells and its relationship to cell density and the cell cycle. Exp Cell Res. 1973 Feb;76(2):390–394. doi: 10.1016/0014-4827(73)90391-1. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Dipasquale A., Pawelek J., McGuire J. S., Lerner A. B. Regulation of melanocyte stimulating hormone action at the receptor level: discontinuous binding of hormone to synchronized mouse melanoma cells during the cell cycle. Proc Natl Acad Sci U S A. 1974 May;71(5):1590–1593. doi: 10.1073/pnas.71.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees J. J., Duell E. A., Bass L. J., Powell J. A., Harrell E. R. Decreased cyclic AMP in the epidermis of lesions of psoriasis. Arch Dermatol. 1972 May;105(5):695–701. [PubMed] [Google Scholar]
- Wong G., Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat New Biol. 1973 Feb 14;241(111):213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J., Sansone M., Morowitz J. Response of mouse melanoma cells to melanocyte stimulating hormone. Nature. 1974 Mar 22;248(446):351–354. doi: 10.1038/248351a0. [DOI] [PubMed] [Google Scholar]
- Yang T. J., Vas S. I. Growth inhibitory effects of adenosine 3',5'-monophosphate on mouse leukemia L-5178-Y-R cells in culture. Experientia. 1971 Apr 15;27(4):442–444. doi: 10.1007/BF02137301. [DOI] [PubMed] [Google Scholar]