Abstract
BACKGROUND
Anemia is a frequent side effect of imatinib in patients with chronic myeloid leukemia (CML). Erythropoietic-stimulating agents have been used for treatment of imatinib-induced anemia. There are no data on long-term safety of erythropoietic-stimulating agents in CML patients.
METHODS
The records of chronic phase CML patients who received treatment with imatinib were reviewed for use of erythropoietic-stimulating agents and occurrence of thrombotic events. Data on cytogenetic response and survival were analyzed by use of erythropoietic-stimulating agent.
RESULTS
A total of 608 patients were included, and 217 patients received erythropoietic-stimulating agents. There were 30 thrombotic episodes. Patients who received erythropoietic-stimulating agents had a higher rate of thrombosis (8.5% vs 2.6%, P = .0025). There was no difference in cytogenetic response rate and survival by use of erythropoietic-stimulating agent. Development of grade 3–4 anemia occurred in 62 (10%) patients and was associated with significantly worse response and survival in patients in late chronic phase. By multivariate analysis, use of erythropoietic-stimulating agents was not a risk factor for event-free survival.
CONCLUSIONS
In our cohort of chronic phase CML patients, use of erythropoietic-stimulating agents did not impact survival or cytogenetic response rate, but was associated with a higher thrombosis rate. Severe anemia is associated with worse survival and response.
Keywords: chronic myeloid leukemia, imatinib, anemia, erythropoiesis-stimulating agents, tyrosine kinase inhibitors
The tyrosine kinase inhibitor (TKI) imatinib (Gleevec; Novartis, Basel, Switzerland) is standard therapy for patients with chronic myeloid leukemia (CML).1 Therapy with imatinib is generally well tolerated, and the most common side effects include nausea, diarrhea, fatigue, muscle cramps, rashes, fluid retention, liver enzymes abnormalities, and myelo-suppression.2,3 Myelosuppression is the most common grade 3–4 toxicity, occurring in 35% to 45% of patients treated with imatinib after interferon (IFN)-α failure and in 15% of patients with previously untreated CML.3–5 Anemia has been reported in 45% to 68% of patients in chronic phase treated with imatinib, and is grade 3-4 in 7% of patients after IFN-α failure and in 3% to 7% of those receiving imatinib as initial CML therapy.3–5 The development of myelosuppression, including anemia, in patients with CML has been associated with an inferior outcome.2,5,6
The use of growth factors such as erythropoietic-stimulating agents (recombinant human erythropoietin [EPO] or darbepoietin-α) and granulocyte-colony stimulating factor during therapy with TKI in CML can improve cytopenias and decrease the frequency of interruptions and dose reductions.6,7 Among patients with chronic phase CML receiving therapy with imatinib, erythropoietic-stimulating agents improved hemoglobin (Hb) levels by at least 2 g/dL in 68% of patients.6
Recently, concerns have been raised regarding the safety of erythropoietic-stimulating agents in patients with cancer.8 A meta-analysis showed that the use of erythropoietic-stimulating agents for prophylaxis or treatment of anemia in patients with cancer increases mortality (hazard ratio [HR], 1.17) and decreases overall survival (OS) (HR, 1.06).9 Another meta-analysis reported that erythropoietic-stimulating agents increase the risk for venous thromboembolic events (relative risk, 1.57) and decrease survival (HR, 1.10) in patients with cancer.10 In addition, a randomized trial showed that use of darbepoietin-α was associated with worse survival in patients with cancer-related anemia not receiving chemotherapy.11 Erythropoietic-stimulating agents shortened time to tumor progression in patients with advanced head and neck cancer receiving radiation therapy when it was administered to target an Hb of >12 g/dL.12 Similarly, use of erythropoietic-stimulating agents decreased OS in patients with metastatic breast cancer and nonsmall cell lung cancer.13,14 This evidence led to a revision in the US Food and Drug Administration product labels for erythropoietic-stimulating agents, issuing a black box warning indicating the possibility of shortened survival and cancer progression in cancer patients treated with erythropoietic-stimulating agents when targeting a Hb level of 10 to 12 g/dL.8
For patients with CML and imatinib-induced anemia, there are few treatment options. The American Society of Hematology/American Society of Clinical Oncology guidelines on the use of erythropoietic-stimulating agents in patients with cancer recommends that for patients with chemotherapy-associated anemia and low-risk myelodysplastic syndrome, erythropoietic-stimulating agents should be initiated as Hb approaches or falls below 10 g/dL to increase Hb and decrease transfusions.8 The dose should be decreased by 25% to 40% when Hb reaches the level needed to avoid transfusion or if the rate of Hb increase is >1 g/dL in 2 weeks.8 However, erythropoietic-stimulating agent are frequently used in patients with CML to treat anemia associated with TKI therapy, but the long-term safety of their use in this setting is unknown.6 We thus conducted this analysis to investigate the prognostic impact of erythropoietic-stimulating agent use among patients with CML in chronic phase treated with imatinib and to define the incidence of cardiovascular, cerebrovascular, or thromboembolic episodes in this patient population.
MATERIALS AND METHODS
Patients
We reviewed the records of 608 consecutive adult patients with a diagnosis of CML in chronic phase who received therapy with single-agent imatinib in phase 2 or 3 studies conducted at The University of Texas M. D. Anderson Cancer Center from December 1999 to December 2005. Patients with clonal evolution but no other criteria for accelerated phase (AP) were also included in this analysis. Patients were enrolled in studies involving CML after IFN-α failure (protocols DM99-367, DM00-139, and ID01-292) or newly diagnosed CML (protocols ID02-534, ID01-151, ID01-015, and DM00-163). In 3 studies (ID01-292, ID02-534, and ID01-151), patients received high-dose imatinib (800 mg daily). In the remaining protocols, patients received standard-dose imatinib (400 mg daily). Details about entry criteria, treatment dose schedules and adjustments, pretreatment, and follow-up evaluations have been detailed in previous reports.3,4,15–19 Studies were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before study entry.
Use of Erythropoietin in Treatment of Anemia
Anemia was graded according to the National Cancer Institute Common Terminology Criteria version 3.0 as grade 1 (<lower limit of normal [LLN]-10 g/dL), grade 2 (<10–8.0 g/dL), grade 3 (<8.0–6.5 g/dL), and grade 4 (<6.5 g/dL). The LLN was 12 g/dL. Use of erythropoietic-stimulating agents was defined as therapy with either EPO or darbepoetin-α concomitant with imatinib therapy, regardless of the cause of the anemia. Indication for use and duration of therapy with erythropoietic-stimulating agents was determined by the treating physician. Patients received different doses and schedules of erythropoietic-stimulating agents at the discretion of the treating physician. The most common starting doses were for EPO 40,000 U once a week, and for darbepoetin-α 200 to 400 μg every 2 weeks. Patients who had signs suggestive of iron deficiency, as determined by clinical history, red blood cell indexes measurements, serum iron, serum total iron binding capacity, and/or serum ferritin concentrations, received oral iron supplementation and were evaluated for possible causes of blood loss before starting on erythropoietic-stimulating agents. Erythropoietin levels were not routinely measured before starting therapy with erythropoietic-stimulating agents.
Patient Monitoring and Response Criteria
Patients were followed with complete blood counts (CBCs) weekly during the first 4 weeks of therapy with imatinib and then every 2 to 4 weeks. Patients who developed myelosuppression had a CBC performed every 1 to 2 weeks. The CML response criteria were as previously published.1 Briefly, a complete hematologic response (CHR) required normalization, for at least 4 weeks, of the bone marrow (BM; <5% blasts) and peripheral blood with a white blood cell (WBC) count <10 × 109/L, without blasts, promyelocytes, or myelocytes, basophils <2%, a platelet count <450 × 109/L, and disappearance of all signs and symptoms of CML. Cytogenetic response was defined by the percentage of Philadelphia chromosome positive (Ph1+) metaphases in at least 20 metaphases from a BM aspirate (fluorescent in situ hybridization if insufficient metaphases) as follows: complete cytogenetic response, 0% Ph1+ metaphases; partial cytogenetic response (partial cytogenetic response)-1%-35% Ph1+ metaphases; minor cytogenetic response, 36% to 65% Ph1+ metaphases; minimal cytogenetic response, 66% to 95% Ph1+ metaphases; no response, 96% to 100% Ph1+ metaphases. Major cytogenetic response was defined as complete cytogenetic response plus partial cytogenetic response (<36% Ph1+ metaphases).
Definition of Event-Free Survival and Overall Survival
Event-free survival (EFS) was defined as the time from start of treatment to the occurrence of an event. An event was defined as death from any cause, transformation to AP or blast phase, loss of major cytogenetic response, and loss of CHR.4 OS was defined from the time treatment was started until death from any cause; patients alive at last follow-up were censored.
Statistical Analysis
Categorical and continuous variables between groups were compared using the chi-square/Fisher exact test and Mann-Whitney U test, respectively.20 Survival curves and probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test.21,22 A logistic regression model was used to evaluate the association between clinical features and development of thrombosis. Variables included in the multivariate logistic regression model were age, sex, use of high-dose imatinib, stage (late chronic phase vs untreated), percentage of Ph1+ cells, WBC, Hb, platelet count, percentage of BM blasts, prior use of IFN-α (yes vs no), Sokal risk (high vs other), duration of disease, and use of erythropoietic-stimulating agents (yes vs no). Variables were eliminated by backward selection, with a P value cutoff of.05. Clinical and biological characteristics were analyzed for their association with EFS using multivariate Cox proportional hazards models.23 Variables significant in the univariate analysis were added to the multivariate model, and nonsignificant variables were eliminated by backward selection with a P value cutoff of.05. The categorical variable use of erythropoietic-stimulating agent was added to the model because it was the variable of interest. All P values were 2-sided. Calculations were done in Statistica, version 6.1 (StatSoft, Tulsa, Okla) and SPSS (SPSS Inc., Chicago, Ill).
RESULTS
Patient Characteristics and Results of Erythropoietic-Stimulating Agent Therapy
A total of 608 patients were analyzed. Three-hundred seven (50%) were in early chronic phase (previously untreated), and 301 (50%) were in late chronic phase (post-IFN-α failure). Forty-one (7%) patients had clonal evolution and were analyzed together with early chronic phase or late chronic phase depending on prior treatment history. High-dose imatinib (800 mg) was used in 249 (41%) patients, whereas the remaining patients received standard-dose imatinib (400 mg). Of those 249 patients, 94 with untreated CML participated in a randomized study of high-dose imatinib alone versus high-dose imatinib combined with IFN-α and sargramostim (granulocyte-macrophage colony-stimulating factor [GM-CSF]), with 45 (7%) patients being randomized to the combination arm.19 Anemia (any degree) was detected during therapy with imatinib in 502 (83%) patients, being grade 3–4 in 62 (10%) patients. Patients treated with high-dose imatinib had a higher incidence of anemia (any grade) than those treated with standard dose (90% vs 78%; P = .0002), but no difference in the incidence of grade 3–4 anemia (11% vs 9%; P = .47). The addition of IFN-α and GM-CSF to high-dose imatinib did not affect the incidence of anemia compared with high-dose imatinib alone (91% vs 82%, P = .11) or grade 3–4 anemia (11% vs 10%, P = .83).
Overall, 217 (36%) patients received therapy with erythropoietic-stimulating agents at some point during treatment with imatinib. Forty (7%) patients were already receiving erythropoietic-stimulating agents before starting therapy with imatinib and continued using erythropoietic-stimulating agents while on imatinib. Among the remaining 177 patients, median time between start of imatinib therapy and start of an erythropoietic-stimulating agent was 9 months (range, 1–89 months). The characteristics of patients who received erythropoietic-stimulating agents versus those who did not are shown in Table 1. Patients who received erythropoietic-stimulating agents were older (57 vs 49 years, P < .0001), had lower Hb at the start of therapy with imatinib (11.7 vs 12.9, P < .0001), and had a higher prevalence of intermediate and high risk by Sokal score (P < .0001). Patients treated with erythropoietic-stimulating agents received therapy with variable, intermittent doses of EPO and/or darbepoetin-α during a median time of 15 months (range, 1–113 months). The median nadir Hb among patients treated with erythropoietic-stimulating agents was 9.2 g/dL (45 [20%] with grade 3–4 anemia) and was 11.1 g/dL (17 [4%] with grade 3–4 anemia) in those not treated with erythropoietic-stimulating agents (P < .0001). The observed best increase in Hb with erythropoietic-stimulating agent therapy was >3 g/dL in 119 (55%), 2 to 3 g/dL in 54 (25%), 1 to <2 g/dL in 33 (15%), and <1 g/dL in 11 (5%) patients. The median Hb level achieved with erythropoietic-stimulating agents was 12.9 g/dL (range, 8.9–16.1 g/dL).
Table 1.
Baseline Characteristics of Patients
| Clinical Feature | Median [Range] or No. (%) | P | |
|---|---|---|---|
| No ESA, n = 391 | ESA, n = 217 | ||
| Age, y | 49 [15–81] | 57 [17–84] | <.0001 |
| Male sex | 258 (66) | 94 (43) | <.0001 |
| Stage | .92 | ||
| ECP | 198 (51) | 109 (50) | |
| LCP | 193 (49) | 108 (50) | |
| Spleen size, cm from left costal margin | 0 [0–20] | 0 [0–25] | .32 |
| WBC, ×109/L | 14.5 [1.6–283] | 18 [1.8–239] | .14 |
| Hb, g/dL | 12.9 [7.9–16.9] | 11.7 [6.2–14.6] | <.0001 |
| Platelets, ×109/L | 288 [58–1476] | 334 [82–1324] | .09 |
| PB blasts | 0 [0–12] | 0 [0–12] | .08 |
| BM blasts | 1 [0–14] | 1 [0–14] | .10 |
| Sokal risk score | <.0001 | ||
| Low | 299 (76) | 124 (57) | |
| Intermediate | 77 (20) | 75 (35) | |
| High | 15 (4) | 18 (8) | |
| Previous IFN-α | 194 (50) | 108 (50) | .97 |
| Ph1+ at start of imatinib >90% | 320 (82) | 189 (88) | .10 |
| Time from diagnosis to imatinib, mo | 8 [0–218] | 6.5 [0–194] | .61 |
| High dose imatinib, % | 155 (40) | 94 (43) | .37 |
ESA indicates erythropoietic-stimulating agent; ECP, early chronic phase; LCP, late chronic phase; WBC, white blood cell count; Hb, hemoglobin; PB, peripheral blood; BM, bone marrow; IFN, interferon; Ph1+, Philadelphia chromosome positive.
Dose Reductions and Erythropoietic-Stimulating Agent Use
Data on imatinib dose reduction were available for 605 patients. There was a higher prevalence of dose reductions (defined as a reduction in dose below the starting dose) in patients who received erythropoietic-stimulating agents versus those who did not (53% vs 38%; P = .0002). We did a subanalysis on only those patients who started with standard-dose imatinib and found similar results (47% vs 25%, P < .00001). However, these results are confounded by the finding that patients who did not develop anemia were less likely to require dose reductions and to have an indication for use of erythropoietic-stimulating agents. Thus, we did an analysis focusing only on 251 patients who developed ≥grade 2 anemia and would have a theoretical indication for erythropoietic-stimulating agent use. In this population of patients, there was no difference in the occurrence of dose reductions by use of erythropoietic-stimulating agents (56% vs 52%, P = .51). Restricting the analysis to those patients who started therapy with standard-dose imatinib also did not demonstrate a statistically significant difference in the rate of dose reductions (53% vs 39%, P = .12).
Thrombotic Episodes and Relationship to Erythropoietic-Stimulating Agent Use
Overall, 27 (4.4%) patients developed 30 thrombotic episodes (3 patients had 2 episodes) after starting therapy with imatinib (Table 2). There were 11 cardiovascular events, 8 cerebrovascular events, and 11 thromboembolic events. Patients had other risk factors for thrombotic disease, including prior history of coronary artery disease (n = 8), concomitant or previous second cancer (n = 5), concomitant thrombocytosis (n = 4), atrial fibrillation (n = 2), and postoperative status for hip surgery (n = 1). Six (20%) events occurred during therapy with erythropoietic-stimulating agents, including 1 episode each of transient ischemic attack, sudden cardiac death, cardiac arrest, myocardial infarction, unstable angina, and pulmonary embolism. The median Hb at the time of event was 10.7 g/dL (range, 8.9–11.4 g/dL). Twelve (40%) episodes occurred in patients who had previously received erythropoietic-stimulating agents but were not receiving erythropoietic-stimulating agents when the event occurred. The median Hb at the time of the event was 11.3 g/dL (range, 9.5–13.5 g/dL), and the median time between discontinuation of erythropoietic-stimulating agent and the thrombotic event was 4 months (range, 1–12 months). Twelve (40%) episodes occurred in patients who were never treated with erythropoietic-stimulating agents. Seventy-two percent of cardiovascular events and 75% of cerebrovascular events occurred in patients with concomitant or prior use of erythropoietic-stimulating agents, whereas 64% of thromboembolic events occurred in patients with no prior history of erythropoietic-stimulating agent use. Overall, the rate of thrombotic events was 8.5% in patients with concomitant or prior use of erythropoietic-stimulating agents versus 2.6% in patients without use of erythropoietic-stimulating agents (P = .0025). In a multivariate logistic regression analysis, the following variables were associated with thrombosis: age (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.01–1.08; P = .003) and use of erythropoietic-stimulating agents (OR, 2.27; 95% CI, 0.98–5.22; P = .05).
Table 2.
Thrombotic Episodes and ESA Use
| Use of ESAs | Number (%) of Events | |||
|---|---|---|---|---|
| Total | Cardiovasculara | Cerebrovascularb | Thromboembolicc | |
| During | 6 (20) | 4 (36) | 1 (12.5) | 1 (9) |
| After | 12 (40) | 4 (36) | 5 (62.5) | 3 (27) |
| No | 12 (40) | 3 (28) | 2 (25) | 7 (64) |
| Total | 30 | 11 | 8 | 11 |
ESA indicates erythropoietic-stimulating agent.
Cardiovascular events: acute myocardial infarction (4 episodes), unstable angina (5 episodes), sudden death and cardiac arrest (1 episode each).
Cerebrovascular events: cerebrovascular accident (6 episodes), transient ischemic attack (2 episodes).
Thromboembolic events: pulmonary embolism (6 episodes), deep venous thrombosis (4 episodes), digital arterial ischemia (1 episode).
Response and Survival
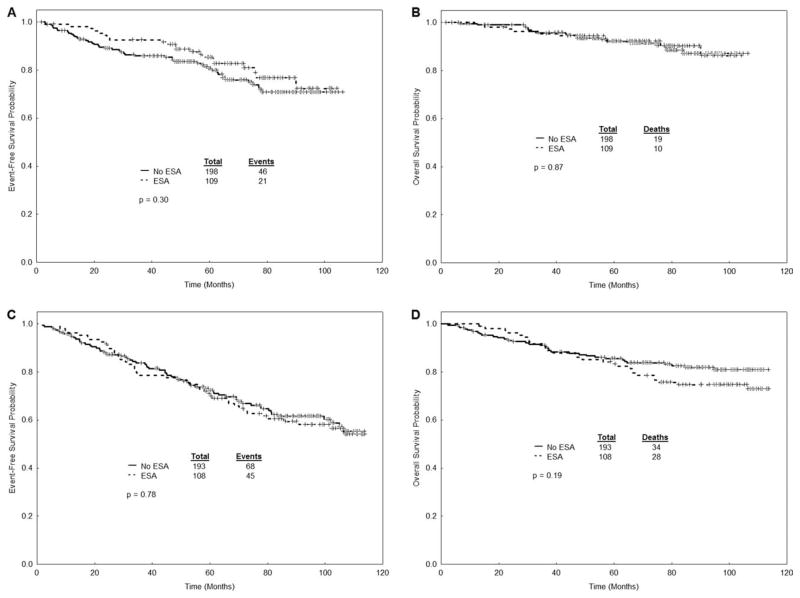
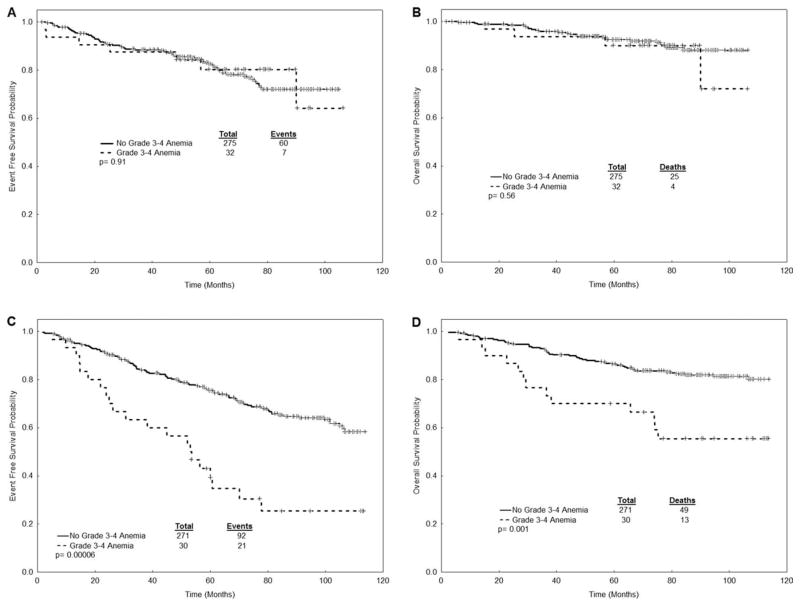
The response and survival outcomes for all patients stratified by disease stage are presented in Table 3 and Figure 1. The median follow-up for the entire cohort was 89 months. There was no statistically significant difference in response rate (major cytogenetic response and complete cytogenetic response), EFS, and OS in patients with CML who received erythropoietic-stimulating agents compared with patients who did not. There was a tendency for higher response rates and higher survival in patients who received erythropoietic-stimulating agents in the subgroup of patients with previously untreated CML. When analyzing only the patients who received standard-dose imatinib (n = 359), there was still no difference in the rates of response and survival (Table 3). Cytogenetic response rates and survival were significantly worse in late chronic phase patients who developed grade 3–4 anemia during treatment with imatinib. Patients in early chronic phase who developed grade 3–4 anemia had worse rates of major cytogenetic response (81% vs 93%), but no differences in EFS and OS (Table 4 and Fig. 2).
Table 3.
Response and Survival by Use of ESAs
| Stage | Use of ESAs | No. | % MCyR | % CCyR | EFS, % at 7 Years (95% CI) | OS, % at 7 Years (95% CI) |
|---|---|---|---|---|---|---|
| All Patients (N=608) | ||||||
| ECP | Yes | 109 | 92a | 91a | 77 (67–87) | 90 (84–97) |
| No | 198 | 91b | 84b | 71 (64–78) | 87 (81–93) | |
| P | .88 | .09 | .30 | .87 | ||
| LCP | Yes | 108 | 76 | 69 | 60 (51–70) | 75 (66–83) |
| No | 193 | 79c | 72c | 62 (54–69) | 82 (76–88) | |
| P | .57 | .59 | .78 | .19 | ||
| Patients who received standard-dose imatinib only (n=359) | ||||||
| ECP | Yes | 24 | 75 | 71 | 60 (40–81) | 83 (68–98) |
| No | 75 | 88d | 78d | 70 (58–81) | 85 (76–94) | |
| P | .12 | .33 | .25 | .43 | ||
| LCP | Yes | 99 | 75 | 68 | 60 (50–70) | 75 (66–83) |
| No | 161 | 75e | 68e | 62 (53–70) | 80 (73–86) | |
| P | .89 | .96 | .76 | .49 | ||
ESA indicates erythropoietic-stimulating agent; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; EFS, event-free survival; CI, confidence interval; OS, overall survival; ECP, early chronic phase; LCP, late chronic phase.
From 108 evaluable patients.
From 193 evaluable patients.
From 188 evaluable patients.
From 72 evaluable patients.
From 159 evaluable patients.
Figure 1.
Survival outcomes are shown according to erythropoietic-stimulating agent (ESA) use: (A) event-free survival (EFS) in early chronic phase patients; (B) overall survival (OS) in early chronic phase patients; (C) EFS in late chronic phase patients; (D) OS in late chronic phase patients.
Table 4.
Response and Survival by Grade 3–4 Anemia
| Stage | Grade 3–4 Anemia | No. | % MCyR | % CCyR | EFS, % at 7 Years (95% CI) | OS, % at 7 Years (95% CI) |
|---|---|---|---|---|---|---|
| ECP | Yes | 32 | 81 | 81 | 80 (66–94) | 90 (79–100) |
| No | 275 | 93a | 87a | 72 (66–78) | 88 (83–93) | |
| P | .03 | .37 | .91 | .56 | ||
| LCP | Yes | 30 | 53 | 40 | 25 (8–42) | 55 (37–74) |
| No | 271 | 80b | 75b | 65 (59–71) | 82 (77–86) | |
| P | .0007 | .0001 | .00006 | .001 |
MCyR indicates major cytogenetic response; CCyR, complete cytogenetic response; EFS, event-free survival; CI, confidence interval; ECP, early chronic phase; LCP, late chronic phase.
From 269 evaluable patients.
From 266 evaluable patients.
Figure 2.
Survival outcomes are shown according to the presence of grade 3–4 anemia: (A) event-free survival (EFS) in early chronic phase patients; (B) overall survival (OS) in early chronic phase patients; (C) EFS in late chronic phase patients; (D) OS in late chronic phase patients.
Multivariate Analysis for EFS
By univariate analysis, the following factors were associated with worse EFS: use of standard-dose imatinib, late chronic phase, higher percentage of Ph1+ cells in BM, higher platelet counts, higher percentage of peripheral blood blasts, higher percentage of BM blasts, older age, development of grade 3–4 anemia, and high-risk Sokal score. By multivariate analysis (Table 5), late chronic phase (vs early chronic phase), higher percentage of Ph1+ cells in BM, higher percentage of BM blasts, high-risk Sokal score, and development of grade 3–4 anemia were associated with worse EFS. Use of erythropoietic-stimulating agents was not associated with worse EFS in the multivariate model.
Table 5.
Multivariate Analysis for EFS
| Parameter | Multivariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | P | |
| LCP (vs ECP) | 2.05 | 1.49–2.83 | <.0001 |
| BM blasts | 1.09 | 1.01–1.16 | .013 |
| Baseline percentage Ph1+ cells in BM | 1.01 | 1.00–1.02 | .011 |
| High Sokal score (vs low/int score) | 2.38 | 1.39–4.05 | .001 |
| Grade 3–4 anemia (yes vs no) | 1.72 | 1.14–2.60 | .009 |
EFS indicates event-free survival; HR, hazard ratio; CI, confidence interval; LCP, late chronic phase; ECP, early chronic phase; BM, bone marrow; Ph1+, Philadelphia chromosome positive; int, intermediate.
DISCUSSION
The use of erythropoietic-stimulating agents in patients with cancer has become a matter of discussion in the past few years, with reports indicating that erythropoietic-stimulating agents may increase the mortality in patients with cancer, particularly when the target Hb is >12 g/dL.8–11 Most of these studies analyzed a heterogeneous group of patients, including both patients with solid tumors and patients with hematological malignancies, and in the majority of reports patients were being treated with conventional chemotherapeutic agents.12–14 Thus, it is important to discern the impact of erythropoietic-stimulating agents in response and survival in different subtypes of cancer and in patients receiving treatment with targeted therapy agents such as TKIs.
We retrospectively analyzed the demographics of use of erythropoietic-stimulating agents in patients with CML treated with imatinib and its impact in response and survival. As expected, patients who used erythropoietic-stimulating agents had a lower median Hb at the start of TKI therapy. Patients who received erythropoietic-stimulating agents also tended to be older, probably a reflection of decreased tolerance to lower Hb levels. Erythropoietic-stimulating agents were highly effective for treatment of anemia in CML. A significant (>2 g/dL) increase in Hb levels was seen in 80% of treated patients. Increases in Hb levels might in theory improve the dose intensity of imatinib and the response rate. However, we did not observe that patients who developed anemia and were subsequently treated with erythropoietic-stimulating agents had a lower rate of dose reductions. Interestingly, there was a trend for a higher complete cytogenetic response rate in patients receiving imatinib as initial therapy who received erythropoietic-stimulating agents.
A previously published meta-analysis linked the use of erythropoietic-stimulating agents with venous thromboembolic events in patients with cancer.10 Most studies analyzed were either focused solely on 1 specific type of solid tumor or excluded myeloid malignancies. CML belongs to the group of myeloproliferative neoplasms, a group of myeloid malignancies that has been known to be associated with thrombotic phenomena.24 We analyzed the prevalence of thrombotic events in this population of patients with CML and its relation with use of erythropoietic-stimulating agents. There was a higher prevalence of thrombotic events in patients who were receiving or had received erythropoietic-stimulating agents, and use of erythropoietic-stimulating agents was associated with thrombosis in a multivariate logistic regression analysis. These events frequently occurred in patients who had other risk factors for thrombotic episodes, such as a prior history of coronary artery disease, and frequently when hemoglobin had increased to 11 g/dL or higher. Thus, firm conclusions regarding the role of erythropoietic-stimulating agents in such thrombotic events cannot be reached. However, clinicians should be cautious with the use of erythropoietic-stimulating agents in CML patients who present with other risk factors for thrombotic events, with particular caution taken not to continue erythropoietic-stimulating agents at Hb levels that approximate 12 g/dL.
In our patient population, use of erythropoietic-stimulating agents did not lead to a decrease in EFS or OS. In a multivariate analysis for EFS, the use of erythropoietic-stimulating agents was not a significant factor. Thus, although concerns may still be raised by the possible association between erythropoietic-stimulating agents and thrombotic events, we could not conclude that erythropoietic-stimulating agents shorten the survival of CML patients being treated with imatinib. This observation should be taken with caution, considering that this was a retrospective analysis of the data and thus could be affected by latent variables not considered in this analysis. Still, there is no obvious adverse effect of the use of erythropoietic-stimulating agents on outcome. Considering the prevalence of anemia in patients treated with TKIs, the effect that anemia has on the quality of life of patients, the chronicity of the therapy, and the lack of alternative options to manage anemia associated with TKI use, the use of erythropoietic-stimulating agents should be more carefully studied to confirm these observations.
The development of severe (grade 3–4) anemia was associated with worse response rates and survival, particularly in patients with late chronic phase CML (post-IFN failure). This is in accordance with previously published reports that demonstrated that the development of anemia in CML patients being treated with imatinib is associated with worse response and survival.5,6,25 This could be a reflection of increased disease burden, as the TKI would induce more profound myelosuppression in patients who had a greater burden of Ph1+ hematopoiesis and insufficient Ph1− hematopoietic cells for a rapid recovery of the BM. Alternatively, it could also be a consequence of dose reductions and treatment interruptions, which could lead to a decrease in the dose intensity and compromise outcomes.26,27 Imatinib also inhibits other tyrosine kinases that are important for hematopoiesis, such as c-Kit,28 and increased plasma levels of imatinib have been associated with development of anemia in CML patients.29
The mechanism by which erythropoietic-stimulating agents may decrease OS in cancer is still unknown. Achieving higher hemoglobin levels may lead to higher mortality from thrombotic events, as has been shown in a study conducted in cardiac patients on hemodialysis receiving erythropoietic-stimulating agents targeted to an Hb level >14 g/dL.30 Cancer cells may express EPO receptors, which are associated with activation of signal-transducing pathways that lead to increased cell survival and proliferation, such as phosphatidylinositol-3-kinase/Akt and Janus kinase/signal transducer and activator of transcription.31–35 An in vitro study has demonstrated that EPO can induce resistance to imatinib in the BCR-ABL1-positive cell line K562.36 However, it must be remembered that K562 is an erythroid cell line, which could have influenced the results observed. In our paper, we did not analyze patients in blast phase, who usually present with a more aggressive phenotype and with additional genetic aberrations, more similar to advanced solid tumors. It is possible that use of erythropoietic-stimulating agents could diminish OS in CML patients in blast phase, but that remains to be proven.
In conclusion, the use of erythropoietic-stimulating agents had no effect on response rate or survival in CML patients in chronic phase receiving imatinib. The prevalence of thrombotic episodes was higher in patients who were receiving or had received erythropoietic-stimulating agents. Development of severe (grade 3–4) anemia was an important risk factor for response and survival in this patient population. Prospective trials studying a larger number of patients are required to clarify the impact of long-term use of erythropoietic-stimulating agents in this patient population.
Footnotes
F.P.S.S. collected data, analyzed data, and wrote the manuscript. Y.A. collected data, analyzed data, and reviewed the manuscript. J.C. designed research, analyzed data, wrote the manuscript, and provided patient care. H.K., D.V., G.M., S.O., F.R., and G.B. provided patient care and reviewed the manuscript.
CONFLICT OF INTEREST DISCLOSURES
Hagop Kantarjian has received research fund support from Novartis. Jorge Cortes has received research fund support from Novartis, BMS, Pfizer and Ortho Biotec. Farhad Ravandi has received honoraria from Novartis and has been a member of advisory boards and speaking bureaus.
References
- 1.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 2.Marin D, Marktel S, Bua M, et al. Prognostic factors for patients with chronic myeloid leukaemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia. 2003;17:1448–1453. doi: 10.1038/sj.leu.2402996. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 5.Sneed TB, Kantarjian HM, Talpaz M, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–121. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, O’Brien S, Quintas A, et al. Erythropoietin is effective in improving the anemia induced by imatinib mesylate therapy in patients with chronic myeloid leukemia in chronic phase. Cancer. 2004;100:2396–2402. doi: 10.1002/cncr.20292. [DOI] [PubMed] [Google Scholar]
- 7.Quintas-Cardama A, De Souza Santos FP, Kantarjian H, et al. Dynamics and management of cytopenias associated with dasatinib therapy in patients with chronic myeloid leukemia in chronic phase after imatinib failure. Cancer. 2009;115:3935–3943. doi: 10.1002/cncr.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update. Blood. 2008;111:25–41. doi: 10.1182/blood-2007-08-109488. [DOI] [PubMed] [Google Scholar]
- 9.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 11.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 13.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 14.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Cortes JE, O’Brien S, et al. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003;101:97–100. doi: 10.1182/blood-2002-02-0545. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 17.Cortes J, Giles F, O’Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian HM, Talpaz M, O’Brien S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: follow-up results. Clin Cancer Res. 2002;8:2177–2187. [PubMed] [Google Scholar]
- 19.Quintas-Cardama A, Kantarjian HM, Ravandi F, et al. Immune modulation of minimal residual disease (MRD) in patients (pts) with chronic myelogenous leukemia (CML) in early chronic phase (CP): A randomized trial of frontline high-dose (HS) imatinib mesylate (IM) with or without pegylated-interferon (PEG-IFN) and GM-CSF [abstract] Blood. 2006;108:Abstract 2207. [Google Scholar]
- 20.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames, IA: Iowa State University Press; 1980. [Google Scholar]
- 21.Kaplan EL, Meire P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Mantel N. Evaluation of survival data and 2 new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 23.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 24.Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22:2020–2028. doi: 10.1038/leu.2008.253. [DOI] [PubMed] [Google Scholar]
- 25.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113:6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes TP, Branford S, White DL, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–3973. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- 27.Jain N, Kantarjian HM, Fava C, et al. Imatinib dose can be safely reduced after complete cytogenetic response (CCyR) in patients (pts) with chronic myeloid leukemia (CML) in early chronic phase (CP) treated with high-dose imatinib [abstract] Blood. 2007;110:Abstract 1043. [Google Scholar]
- 28.Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- 29.Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 30.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 31.Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 32.Lai SY, Childs EE, Xi S, et al. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442–4449. doi: 10.1038/sj.onc.1208635. [DOI] [PubMed] [Google Scholar]
- 33.Mohyeldin A, Lu H, Dalgard C, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7:537–543. doi: 10.1593/neo.04685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardee ME, Rabbani ZN, Arcasoy MO, et al. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther. 2006;5:356–361. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- 35.Kumar SM, Yu H, Fong D, Acs G, Xu X. Erythropoietin activates the phosphoinositide 3-kinase/Akt pathway in human melanoma cells. Melanoma Res. 2006;16:275–283. doi: 10.1097/01.cmr.0000222594.60611.c3. [DOI] [PubMed] [Google Scholar]
- 36.Kirschner KM, Baltensperger K. Erythropoietin promotes resistance against the Abl tyrosine kinase inhibitor imatinib (STI571) in K562 human leukemia cells. Mol Cancer Res. 2003;1:970–980. [PubMed] [Google Scholar]