Abstract
Normal and immune sera from various animal species were fractionated on columns of Sepharose covalently coupled with the glycoprotein fetuin. Elution of the material bound to fetuin yielded low but reproducible amounts of protein, ranging from 0.02 to 0.2% of the protein mass of the input sera. This material has been identified by immunoelectrophoresis in agar and by zone electrophoresis on cellulose acetate as immunoglobulin. The Ig fractions bound and agglutinated erythrocytes of various species, and also bound to cells from various mouse tissues including heart, kidney, thymus, and spleen. In all cases, the binding was inhibited by glycoproteins such as fetuin and thyroglobulin, by a glycopeptide isolated from fetuin, and by some bacterial lipopolysaccharides. When the binding of these Ig fractions to mouse splenocytes was tested in the presence of 17 saccharides, no inhibition of binding was observed except by sialic acid, D-galactose, N-acetyl-D-glucosamine, and D-mannose, all of which showed partial inhibition. Inasmuch as these four saccharides are present on the carbohydrate moiety of fetuin, the results suggest that the isolated material is a carbohydrate-specific Ig (CS-Ig) fraction of serum capable of binding to the carbohydrate portion of cell surface receptors and glycoproteins. When bound to lymphocytes, these CS-Ig molecules induced redistribution (patching and capping) of cell surface receptors. Moreover, the CS-Ig fractions from chicken and rabbit sera were weakly mitogenic for mouse splenic lymphocytes. CS-Ig fractions are useful new reagents for studying glycoproteins and the interactions and activities of cell surface carbohydrates.
Full text
PDF
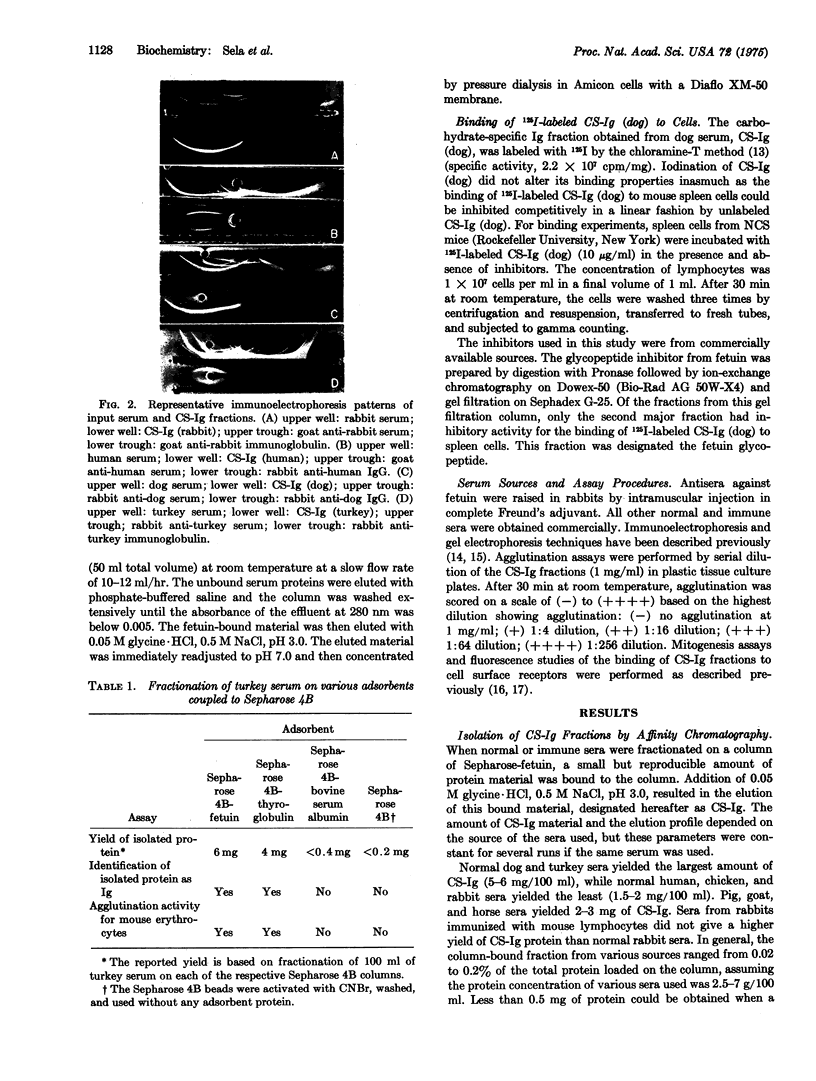

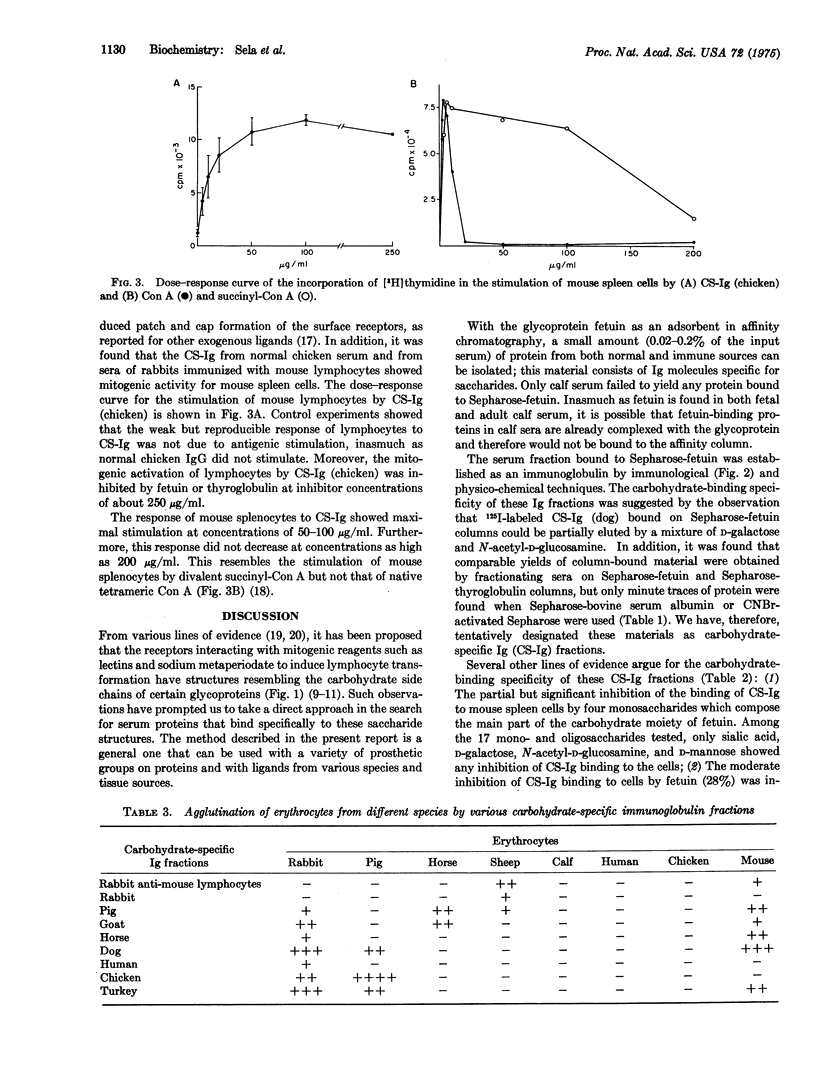

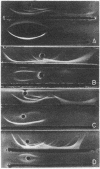
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMANN F. H., LEVINE L., SPIRO R. G. Fetuin: immunochemistry and quantitative estimation in serum. Biochim Biophys Acta. 1962 Mar 26;58:41–51. doi: 10.1016/0006-3002(62)90815-6. [DOI] [PubMed] [Google Scholar]
- Chase P. S., Miller F. Preliminary evidence for the structure of the concanavalin-A binding site on human lymphocytes that induces mitogenesis. Cell Immunol. 1973 Jan;6(1):132–139. doi: 10.1016/0008-8749(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Edelman G. M. The kinetics of cellular commitment during stimulation of lymphocytes by lectins. J Cell Biol. 1974 Aug;62(2):366–377. doi: 10.1083/jcb.62.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Merler E., Gatien J., DeWilde G. Significance of immunofluorescent staining of lymphocytes with antisera to IgM immunoglobulins. Nature. 1974 Oct 18;251(5476):652–654. doi: 10.1038/251652a0. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Induction of lymphocyte transformation by sequential treatment with neuraminidase and galactose oxidase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1824–1827. doi: 10.1073/pnas.70.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Plocinik B. A. Carbohydrate inhibition studies of the naturally occurring human antibody to neuraminidase-treated human lymphocytes. J Immunol. 1974 Sep;113(3):848–858. [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A revised structure for the Forssman glycolipid hapten. J Biol Chem. 1971 Sep 25;246(18):5766–5769. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- TURK J. L. The immune-adherence activity of normal serum. Br J Exp Pathol. 1959 Apr;40(2):97–106. [PMC free article] [PubMed] [Google Scholar]
- Toyoshima S., Fukuda M., Osawa T. Chemical nature of the receptor site for various phytomitogens. Biochemistry. 1972 Oct 10;11(21):4000–4005. doi: 10.1021/bi00771a025. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]