Abstract
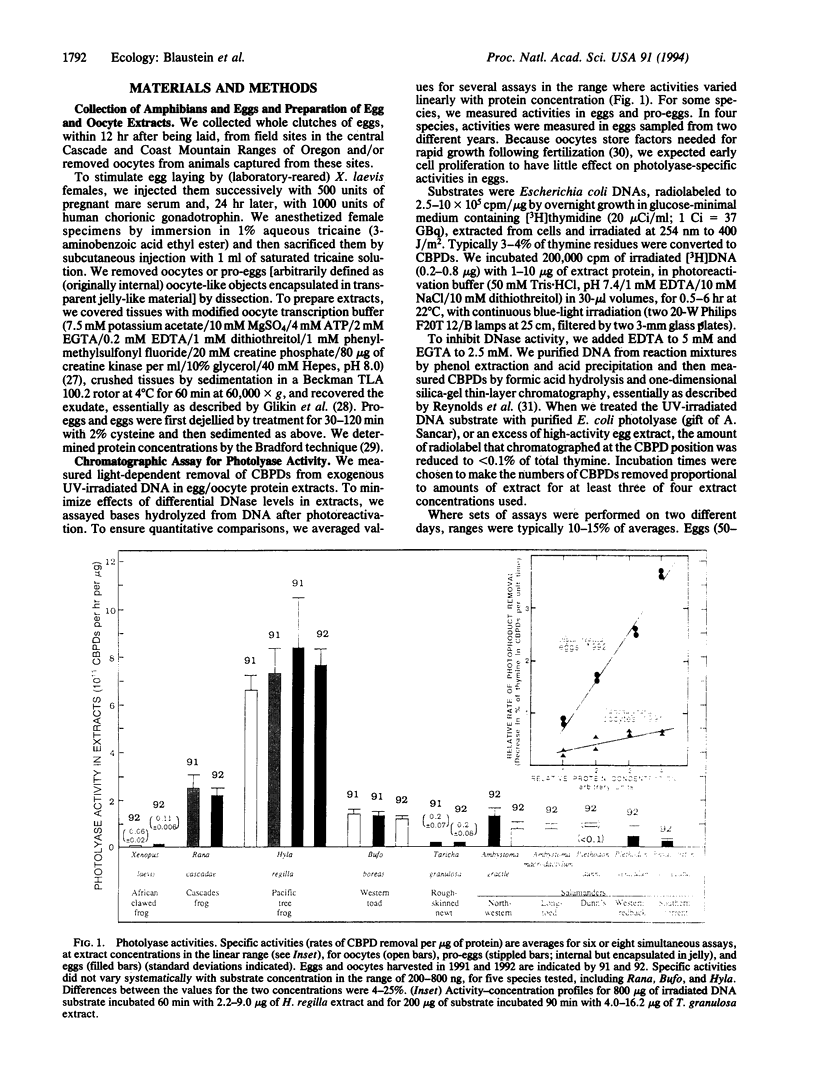
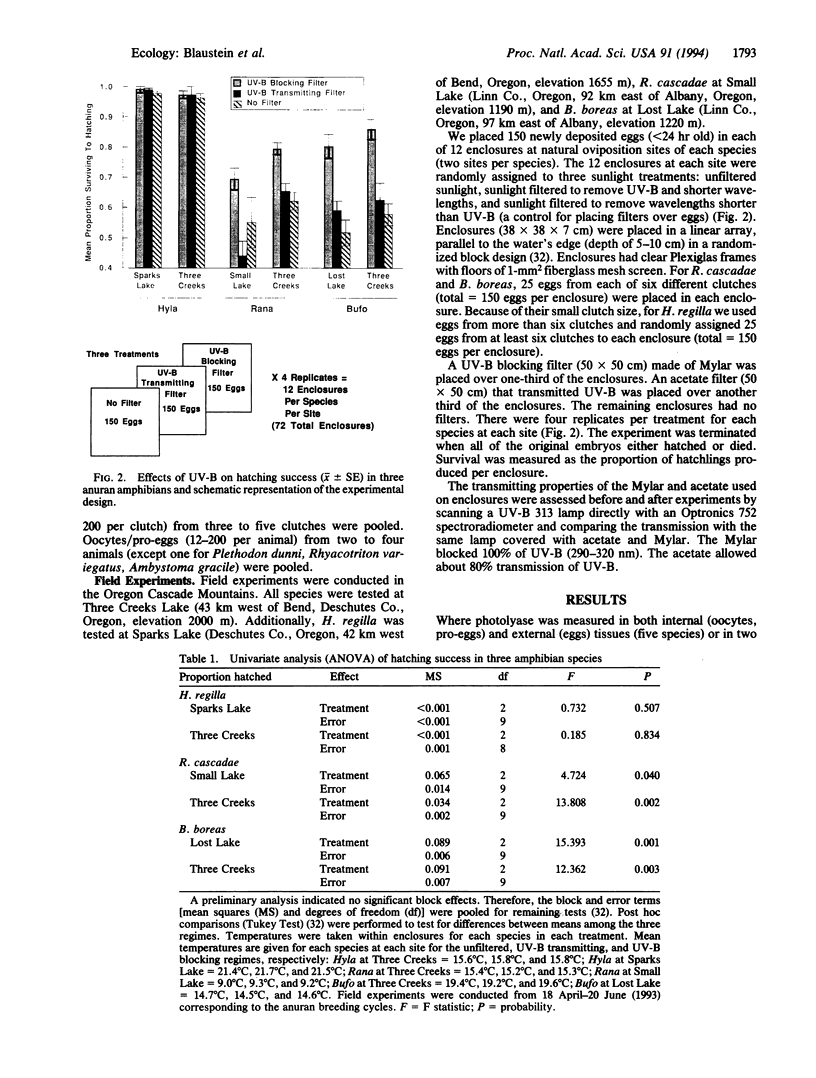
The populations of many amphibian species, in widely scattered habitats, appear to be in severe decline; other amphibians show no such declines. There is no known single cause for the declines, but their widespread distribution suggests involvement of global agents--increased UV-B radiation, for example. We addressed the hypothesis that differential sensitivity among species to UV radiation contributes to these population declines. We focused on species-specific differences in the abilities of eggs to repair UV radiation damage to DNA and differential hatching success of embryos exposed to solar radiation at natural oviposition sites. Quantitative comparisons of activities of a key UV-damage-specific repair enzyme, photolyase, among oocytes and eggs from 10 amphibian species were reproducibly characteristic for a given species but varied > 80-fold among the species. Levels of photolyase generally correlated with expected exposure of eggs to sunlight. Among the frog and toad species studied, the highest activity was shown by the Pacific treefrog (Hyla regilla), whose populations are not known to be in decline. The Western toad (Bufo boreas) and the Cascades frog (Rana cascadae), whose populations have declined markedly, showed significantly lower photolyase levels. In field experiments, the hatching success of embryos exposed to UV radiation was significantly greater in H. regilla than in R. cascadae and B. boreas. Moreover, in R. cascadae and B. boreas, hatching success was greater in regimes shielded from UV radiation compared with regimes that allowed UV radiation. These observations are thus consistent with the UV-sensitivity hypothesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumthaler M., Ambach W. Indication of increasing solar ultraviolet-B radiation flux in alpine regions. Science. 1990 Apr 13;248(4952):206–208. doi: 10.1126/science.2326634. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cook J. S., McGrath J. R. Photoreactivating-enzyme activity in metazoa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1359–1365. doi: 10.1073/pnas.58.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Kerr J. B., McElroy C. T. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science. 1993 Nov 12;262(5136):1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- Lund E., Paine P. L. Nonaqueous isolation of transcriptionally active nuclei from Xenopus oocytes. Methods Enzymol. 1990;181:36–43. doi: 10.1016/0076-6879(90)81110-g. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Pang Q., Hays J. B. UV-B-Inducible and Temperature-Sensitive Photoreactivation of Cyclobutane Pyrimidine Dimers in Arabidopsis thaliana. Plant Physiol. 1991 Feb;95(2):536–543. doi: 10.1104/pp.95.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann J. H., Scott D. E., Semlitsch R. D., Caldwell J. P., Vitt L. J., Gibbons J. W. Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science. 1991 Aug 23;253(5022):892–895. doi: 10.1126/science.253.5022.892. [DOI] [PubMed] [Google Scholar]
- Stolarski R., Bojkov R., Bishop L., Zerefos C., Staehelin J., Zawodny J. Measured trends in stratospheric ozone. Science. 1992 Apr 17;256(5055):342–349. doi: 10.1126/science.256.5055.342. [DOI] [PubMed] [Google Scholar]
- Wake D. B. Declining amphibian populations. Science. 1991 Aug 23;253(5022):860–860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- Worrest R. C., Kimeldorf D. J. Distortions in amphibian development induced by ultraviolet-B enhancement (290-315 NM) of a simulated solar spectrum. Photochem Photobiol. 1976 Oct;24(4):377–382. doi: 10.1111/j.1751-1097.1976.tb06840.x. [DOI] [PubMed] [Google Scholar]
- Worrest R. C., Kimeldorf D. J. Photoreactivation of potentially lethal, UV-induced damage to boreal toad (Bufo boreas boreas) tadpoles. Life Sci. 1975 Nov 15;17(10):1545–1550. doi: 10.1016/0024-3205(75)90175-7. [DOI] [PubMed] [Google Scholar]