Abstract
Gene 32 of bacteriophage T4 is essential for DNA replication, recombination, and repair. In an attempt to clarify the role of the corresponding gene product, we have looked for mutations that specifically inactivate one but not all of its functions and for compensating suppressor mutations in other genes. Here we describe a gene 32 ts mutant that does not produce progeny, but in contrast to an am mutant investigated by others, is capable of some primary and secondary DNA replication and of forming "joint" recombinational intermediates after infection of Escherichia coli B at the restrictive temperature. However, parental and progeny DNA strands are not ligated to covalently linked "recombinant" molecules, and single strands of vegetative DNA do not exceed unit length. Progeny production as well as capacity for covalent linkage in this gene 32 ts mutant are partially restored by additional rII mutations. Suppression by rII depends on functioning host ligase [EC 6.5.1.2; poly(deoxyribonucleotide):poly(deoxyribonucleotide) ligase (AMP-forming, NMN-forming)]. This gene 32 ts mutation (unlike some others) in turn suppresses the characteristic plaque morphology of rII mutants. We conclude that gene 32 protein, in addition to its role in DNA replication and in the formation of "joint" recombinational intermediates, interacts with T4 ligase [EC 6.5.1.1; poly(deoxyribonucleotide):poly(deoxyribonucleotide) ligase (AMP-forming)] when recombining DNA strands are covalently linked. The protein of the mutant that we describe here is mainly defective in this interaction, thus inactivating T4 ligase in recombination. Suppressing rII mutations facilitate substitution of host ligase. There is suggestive evidence that these interactions occur at the membrane.
Full text
PDF

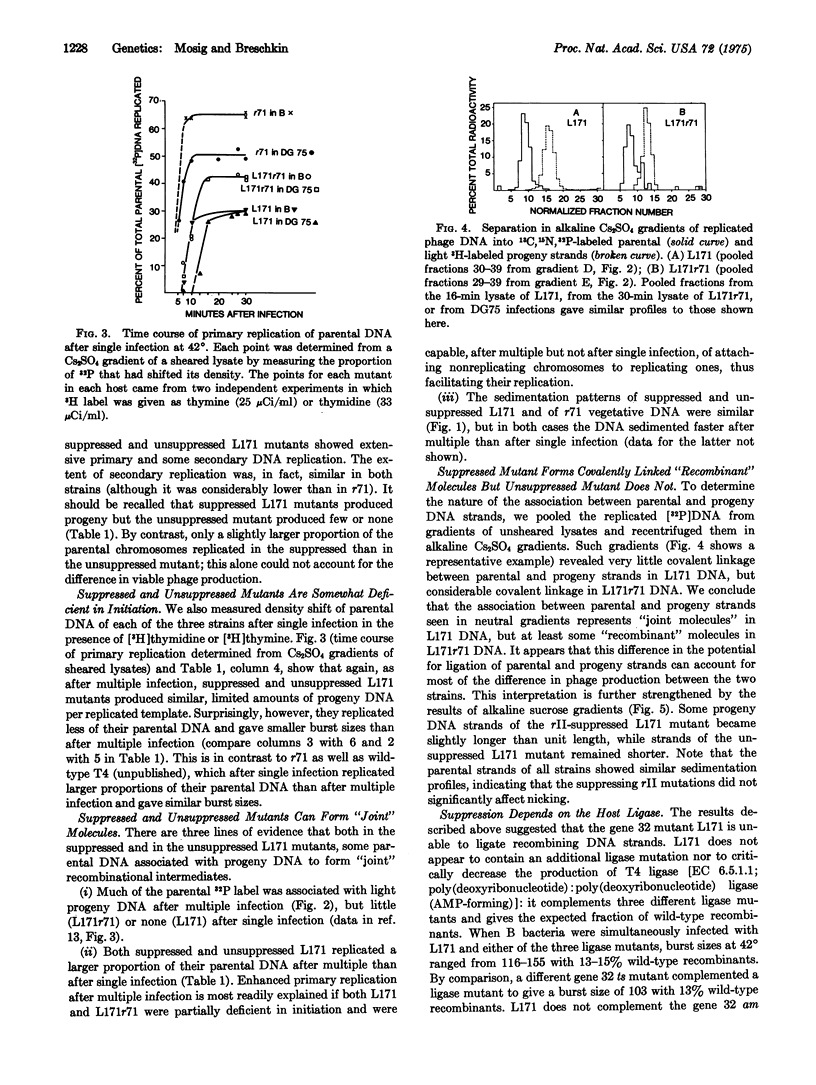
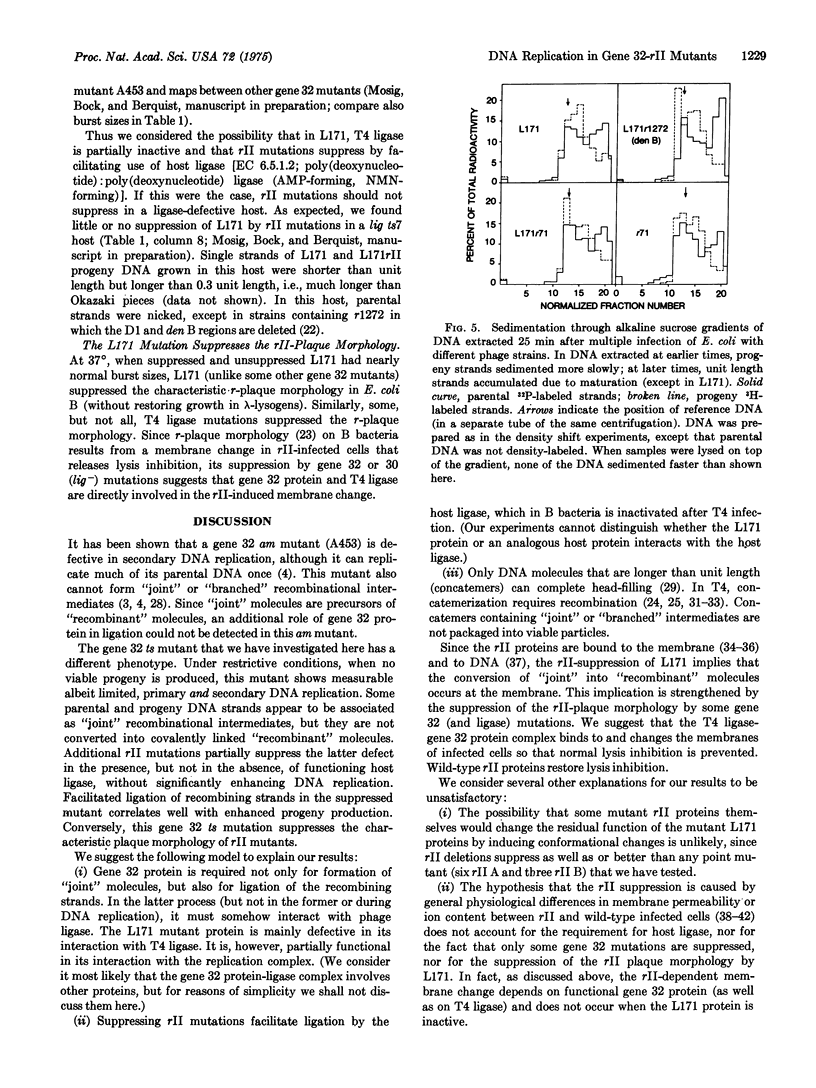

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- BROCK M. L. THE EFFECTS OF POLYAMINES ON THE REPLICATION OF T4RII MUTANTS IN ESCHERICHIA COLI K-12 (LAMBDA). Virology. 1965 Jun;26:221–227. doi: 10.1016/0042-6822(65)90049-8. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Ebisuzaki K., Campbell L. On the role of ligase in genetic recombination in bacteriophage T4. Virology. 1969 Aug;38(4):701–703. doi: 10.1016/0042-6822(69)90190-1. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Kievitt K. D. Association of the rIIA protein with the bacterial membrane. Proc Natl Acad Sci U S A. 1973 May;70(5):1468–1472. doi: 10.1073/pnas.70.5.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Krylov V. N. A mutation of T4B phage, which enhances suppression of ligase mutants with rII mutations. Virology. 1972 Oct;50(1):291–293. doi: 10.1016/0042-6822(72)90375-3. [DOI] [PubMed] [Google Scholar]
- Little J. W. Mutants of bacteriophage T4 which allow amber mutants of gene 32 to grow in ochre-suppressing hosts. Virology. 1973 May;53(1):47–59. doi: 10.1016/0042-6822(73)90464-9. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Breschkin A. M., Mosig G. Origin and direction of bacteriophage T4 DNA replication. II. A gradient of marker frequencies in partially replicated T4 DNA as assayed by transformation. J Mol Biol. 1971 Sep 14;60(2):213–233. doi: 10.1016/0022-2836(71)90289-0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Bowden D. W., Bock S. E. coli DNA polymerase I and other host functions participate in T4 DNA replication and recombination. Nat New Biol. 1972 Nov 1;240(96):12–16. doi: 10.1038/newbio240012a0. [DOI] [PubMed] [Google Scholar]
- Mosig G. Recombination in bacteriophage T4. Adv Genet. 1970;15:1–53. doi: 10.1016/s0065-2660(08)60070-x. [DOI] [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Bacteriophage-induced functions in Escherichia coli K (lambda) infected with rII mutants of bacteriophage T4. J Bacteriol. 1966 Jan;91(1):76–80. doi: 10.1128/jb.91.1.76-80.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Sinha N. K., Snustad D. P. DNA synthesis in bacteriophage T4-infected Escherichia coli: evidence supporting a stoichiometric role for gene 32-product. J Mol Biol. 1971 Nov 28;62(1):267–271. doi: 10.1016/0022-2836(71)90145-8. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. I. Molecular mechanisms of genetic recombination in bacteriophage: joint molecules and their conversion to recombinant molecules. J Cell Physiol. 1967 Oct;70(2 Suppl):201–213. doi: 10.1002/jcp.1040700414. [DOI] [PubMed] [Google Scholar]
- Vetter D., Sadowski P. D. Point mutants in the D2a region of bacteriophage T4 fail to induce T4 endonuclease IV. J Virol. 1974 Aug;14(2):207–213. doi: 10.1128/jvi.14.2.207-213.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernagel W., Radding C. M. Formation in vitro of infective joint molecules of lambda DNA by T4 gene-32 protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):431–435. doi: 10.1073/pnas.71.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]
- Werner R. Initiation and propagation of growing points in the DNA of phage T4. Cold Spring Harb Symp Quant Biol. 1968;33:501–507. doi: 10.1101/sqb.1968.033.01.058. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. Requirement of a functional gene 32 product of bacteriophage T4 in UV, repair. J Virol. 1973 Oct;12(4):758–765. doi: 10.1128/jvi.12.4.758-765.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


