Abstract
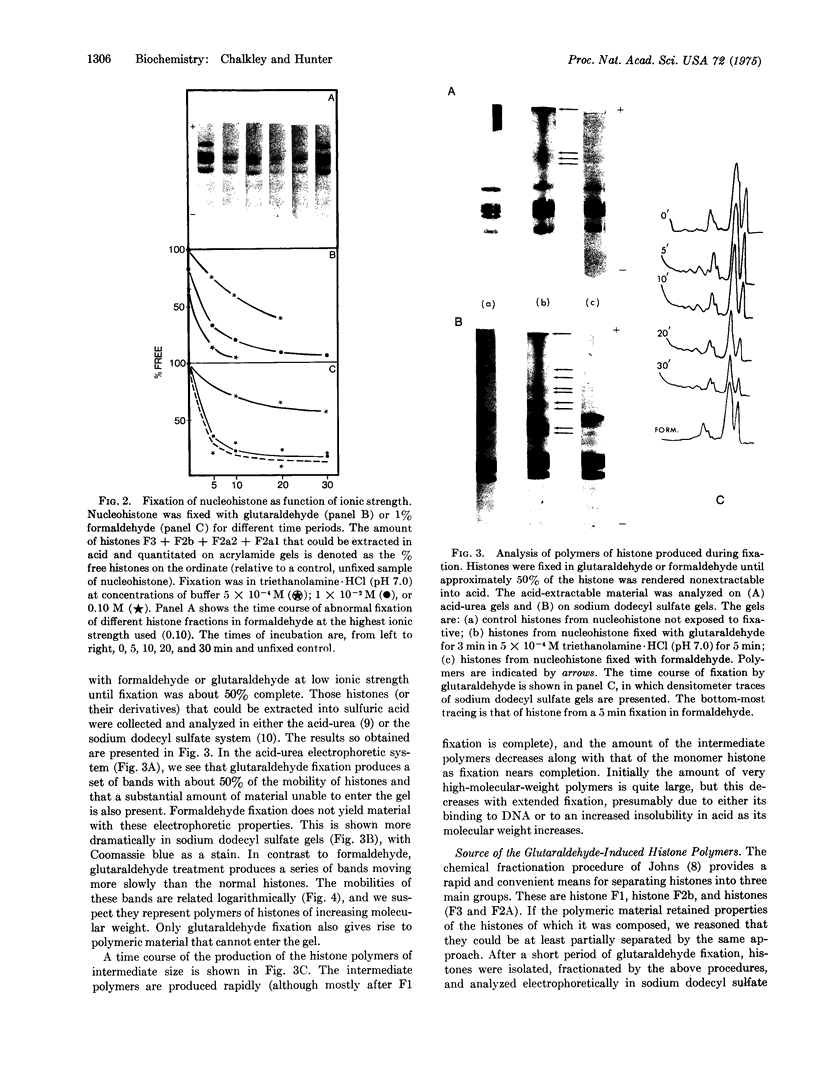
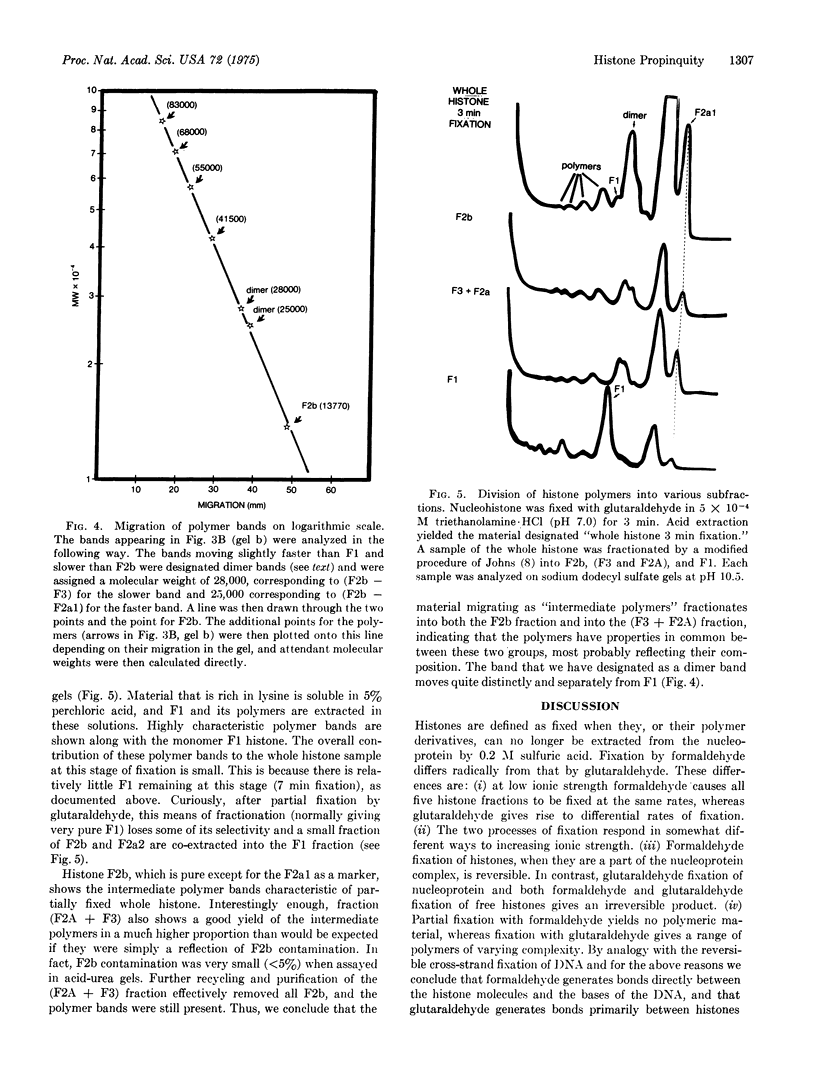
Histones have been fixed within the chromatin complex using either formaldehyde or glutaraldehyde. Evidence is presented which argues that in short time periods formaldehyde fixation leads to the formation of reversible covalent bonds between histone and DNA.On the other hand, fixation of chromatin with glutaraldehyde leads initially to the formation of polymers of F1 histone, and at a later stage of multiple small oligomers of the remaining histones. There oligomers then increase in size until they become too large to detect by polyacrylamide gel electrophoresis. Exclusive formation of histone dimers or tetramers was not observed. The simplest model for histone distribution on DNA which encompasses these observations is one in which histones are organized as a fairly extensive linear overlapping array.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Schlehuber C., Bonner J. Properties of formaldehyde-treated nucleohistone. Biochemistry. 1969 Aug;8(8):3214–3218. doi: 10.1021/bi00836a013. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Separation of newly synthesized nucleohistone by equilibrium centrifugation in cesium chloride. Biochemistry. 1974 Sep 10;13(19):3952–3956. doi: 10.1021/bi00716a021. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan L., Kaltschmidt E. Glutaraldehyde reactivity of the proteins of Escherichia coli ribosomes. Biochemistry. 1972 Jul 4;11(14):2691–2698. doi: 10.1021/bi00764a022. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Nancock R. Separation by equilibrium centrifugation in CsC1 gradients of density--labelled and normal deoxyribonucleoprotein from chromatin. J Mol Biol. 1970 Mar 14;48(2):357–360. doi: 10.1016/0022-2836(70)90167-1. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Wright E. B. Glutaraldehyde fixation of isolated eucaryotic nuclei. Evidence for histone-histone proximity. J Cell Biol. 1973 Nov;59(2 Pt 1):304–317. doi: 10.1083/jcb.59.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J Biol Chem. 1971 Dec 25;246(24):7557–7560. [PubMed] [Google Scholar]
- Subramanian A. R. Glutaraldehyde fixation of ribosomes. Its use in the analysis of ribosome dissociation. Biochemistry. 1972 Jul 4;11(14):2710–2714. doi: 10.1021/bi00764a025. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Ilyin Y. V., Georgiev G. P. Very long stretches of free DNA in chromatin. Nature. 1974 Aug 16;250(467):602–606. doi: 10.1038/250602a0. [DOI] [PubMed] [Google Scholar]