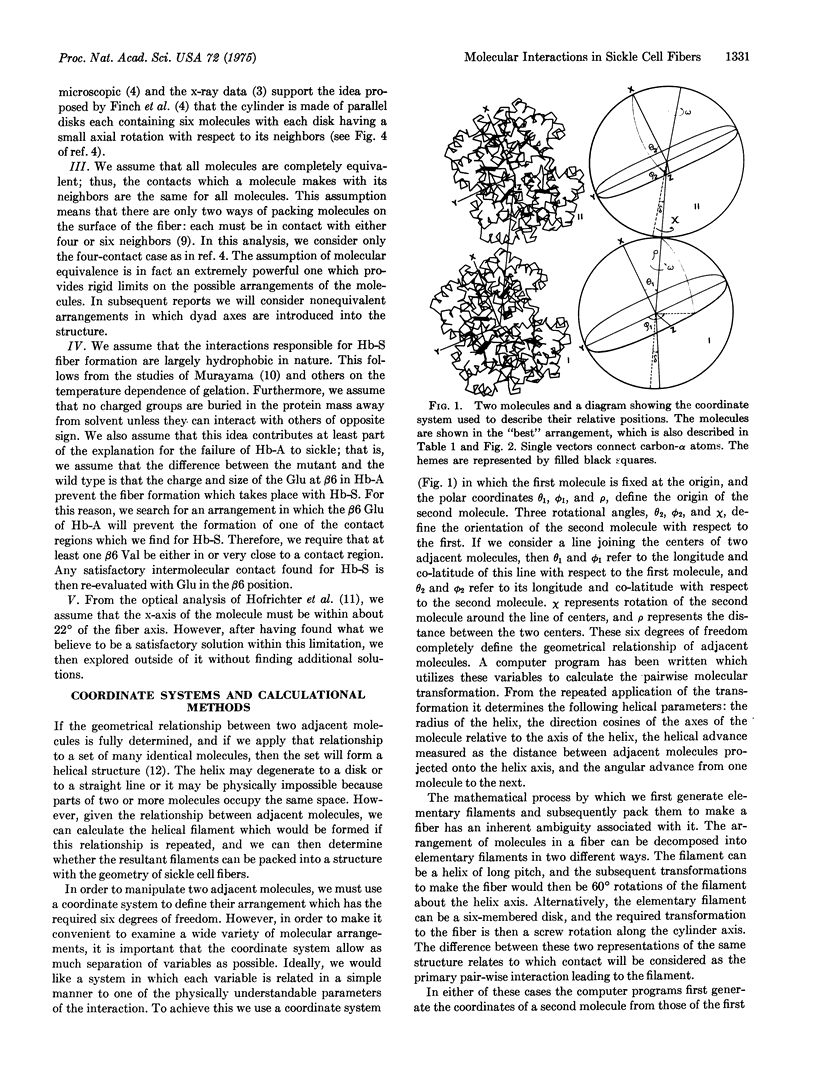
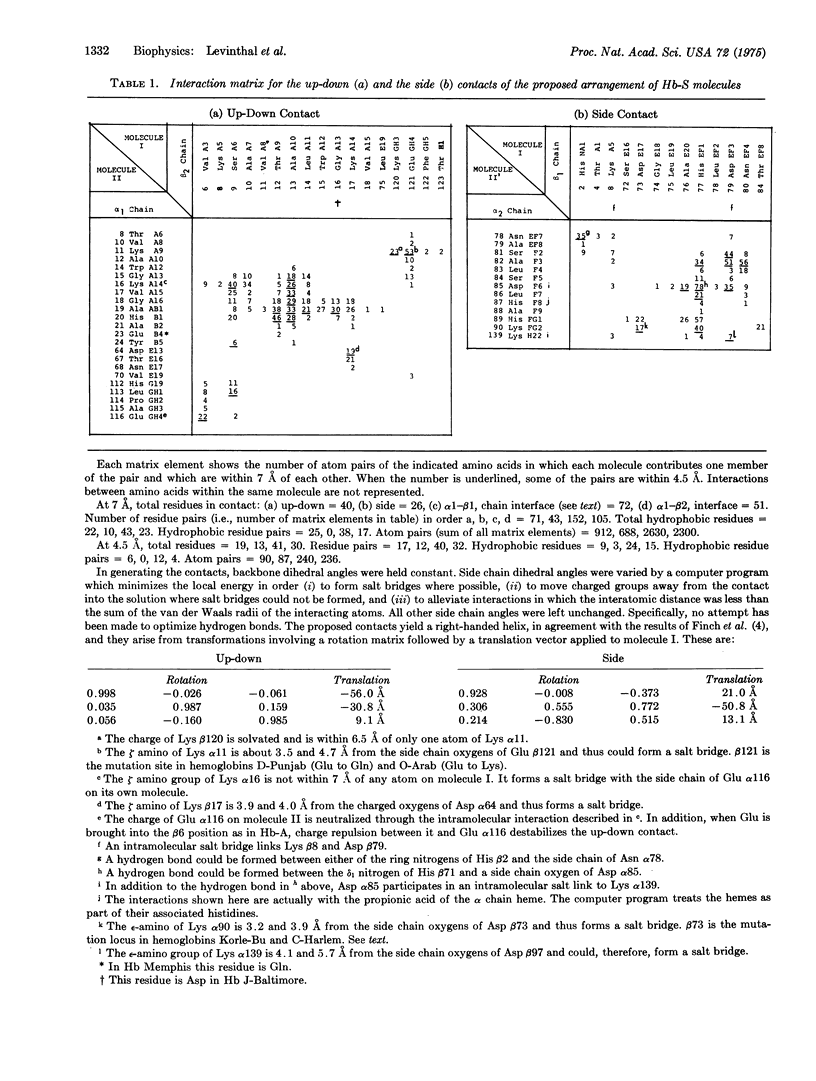
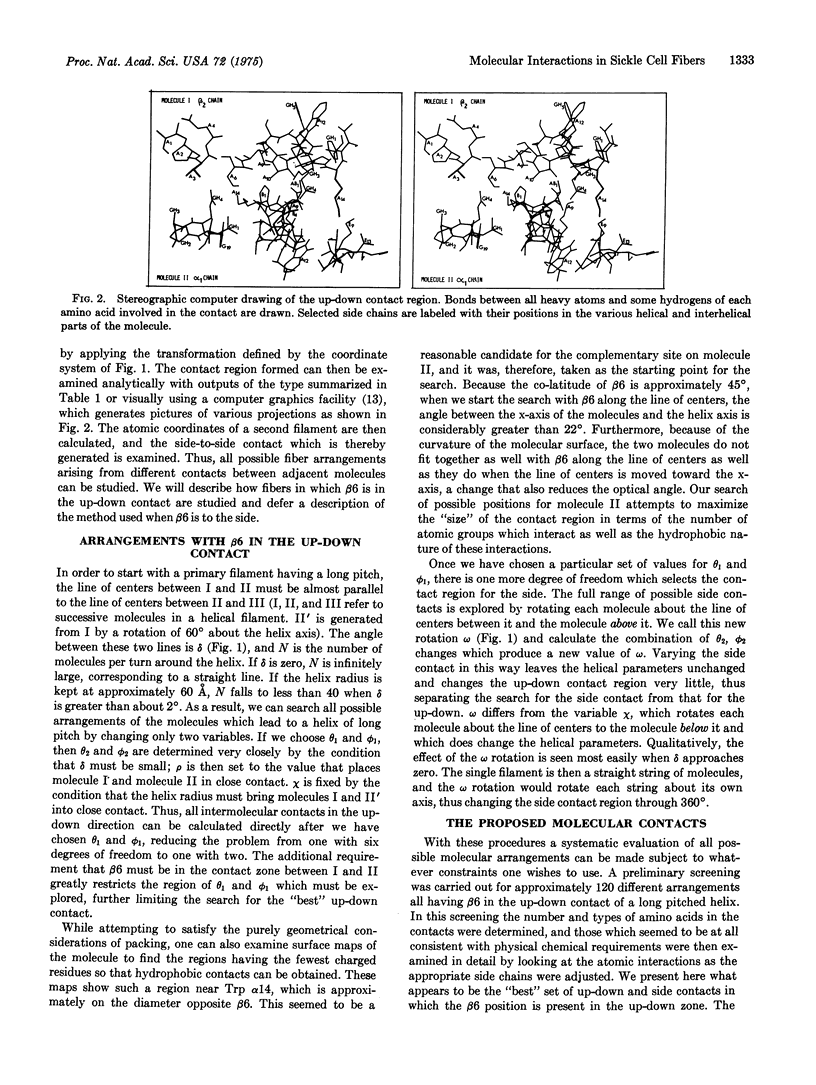
Abstract
Computerized molecular model building has been used to deduce the arrangement of sickle cell hemoglobin molecules (Hb-S) in the tubular fibers which form within sickling cells and in concentrated cell-free solutions of deoxygenated Hb-S. A "best" solution has been found which satisfies all of the reported properties of these fibers. In the proposed arrangement the contact between adjacent Hb-S molecules in the direction parallel to the fiber axis is primarily hydrophobic and in addition contains two salt bridges between the molecules. This contact would be disrupted with the Glu of Hb-A at the beta6 position instead of the Val of Hb-S, and it would not make a long fiber with oxygenated Hb-S. Residues in the A helix and the GH corner of the beta2 chain of one molecule are in contact with residues of the A, B, and E helices and the GH corner of the alpha1 chain of its neighbor. The intermolecular contact in the direction perpendicular to the fiber axis is mainly between the end of the E helix and the EF corner of the beta1 chain on the first molecule and the F helix and FG corner of the alpha2 chain of its neighbor. Some of the implications of these contacts are reported here, and others will be presented in subsequent papers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. The effect of beta 73 Asn on the interactions of sickling hemoglobins. Biochim Biophys Acta. 1970 Nov 17;221(2):373–375. doi: 10.1016/0005-2795(70)90279-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Hendricker D. G., Eaton W. A. Structure of hemoglobin S fibers: optical determination of the molecular orientation in sickled erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3604–3608. doi: 10.1073/pnas.70.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. III. The chemical difference between normal and sickle cell haemoglobins. Biochim Biophys Acta. 1959 Dec;36:402–411. doi: 10.1016/0006-3002(59)90183-0. [DOI] [PubMed] [Google Scholar]
- Katz L., Levinthal C. Interactive computer graphics and representation of complex biological structures. Annu Rev Biophys Bioeng. 1972;1:465–504. doi: 10.1146/annurev.bb.01.060172.002341. [DOI] [PubMed] [Google Scholar]
- MURAYAMA M. Titratable sulfhydryl groups of normal and sickle cell hemoglobins at O degrees and 38 degrees. J Biol Chem. 1957 Sep;228(1):231–240. [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- PERUTZ R. R., LIQUORI A. M., EIRICH F. X-ray and solubility studies of the haemoglobin of sickle-cell anaemia patients. Nature. 1951 Jun 9;167(4258):929–931. doi: 10.1038/167929a0. [DOI] [PubMed] [Google Scholar]


