Abstract
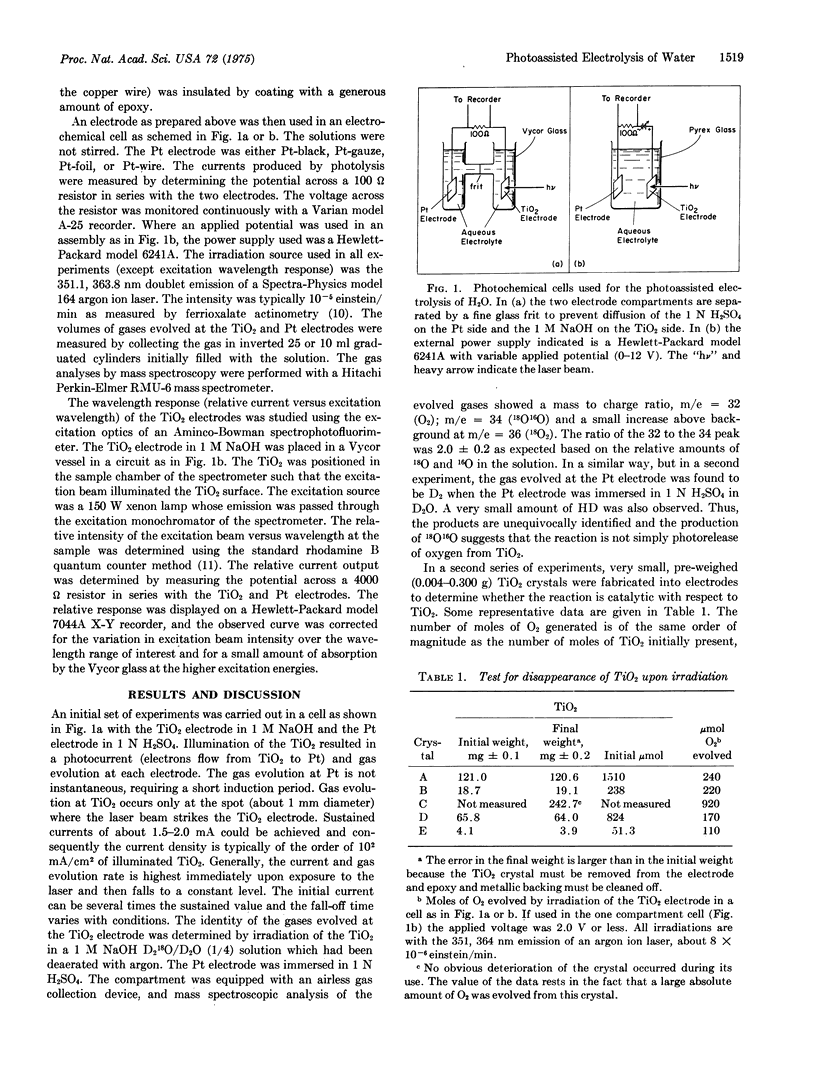
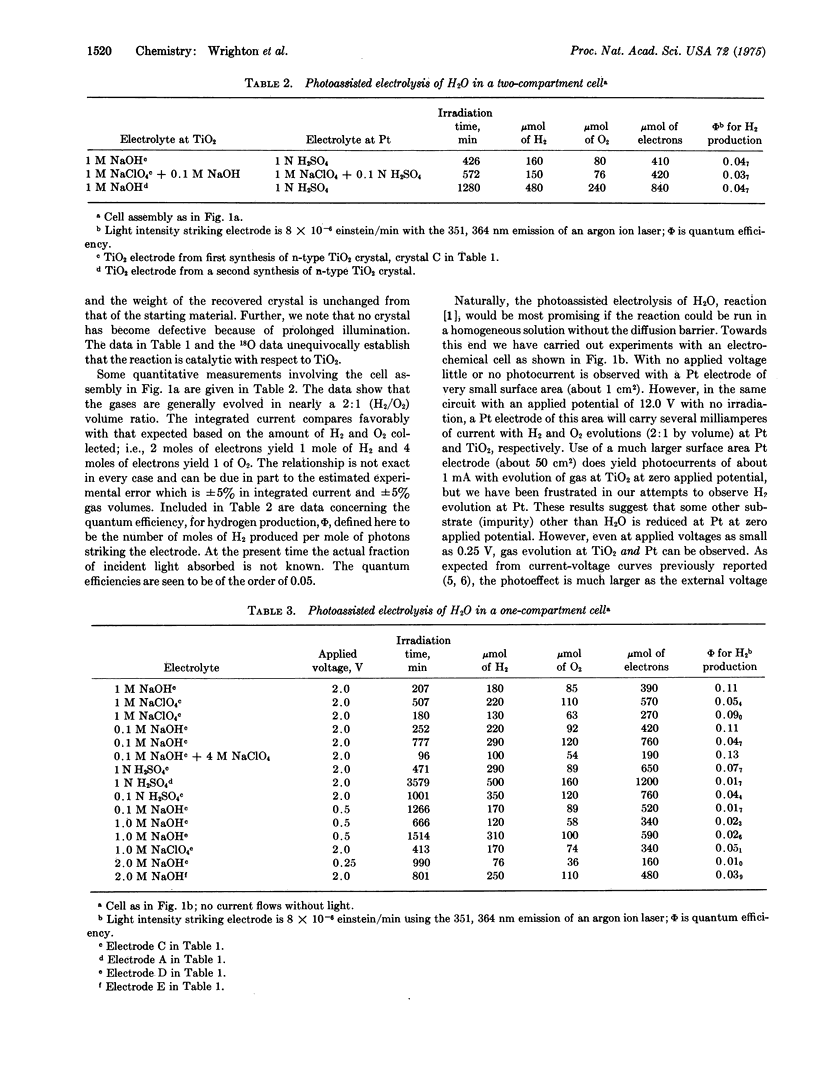
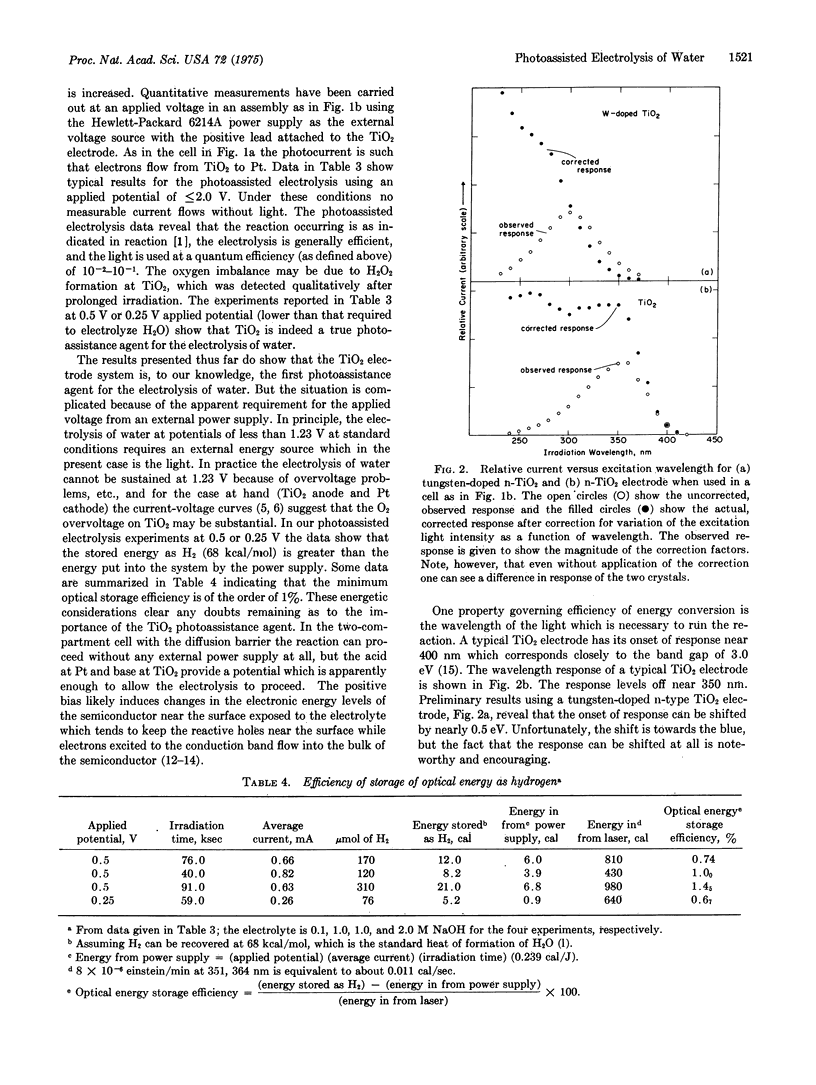
Ultraviolet irradiation (351, 364 nm) of the n-type semiconductor TiO2 as the single crystal electrode of an aqueous electrochemical cell evolves O2 at the TiO2 electrode and H2 at the Pt electrode. The gases are typically evolved in a two: one (H2:O2) volume ratio. The photoassisted reaction seems to require applied voltages, but values as low as 0.25 V do allow the photoassisted electrolysis to proceed. Prolonged irradiation in either acid or base evolves the gaseous products in amounts which clearly demonstrate that the reaction is catalytic with respect to the TiO2. The wavelength response of the TiO2 and the correlation of product yield and current are reported. The results support the claim that TiO2 is a true photoassistance agent for the electrolysis of water. Minimum optical storage efficiencies of the order of 1% can be achieved by the production of H2.
Keywords: optical energy conversion, hydrogen production, photoelectrolysis, solar energy
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- HEIDT L. J., MCMILLAN A. F. [Conversion of sunlight into chemical energy available in storage for man's use]. Science. 1953 Jan 23;117(3030):75–76. doi: 10.1126/science.117.3030.75. [DOI] [PubMed] [Google Scholar]
- Marcus R. J. Chemical Conversion of Solar Energy. Science. 1956 Mar 9;123(3193):399–405. doi: 10.1126/science.123.3193.399. [DOI] [PubMed] [Google Scholar]


