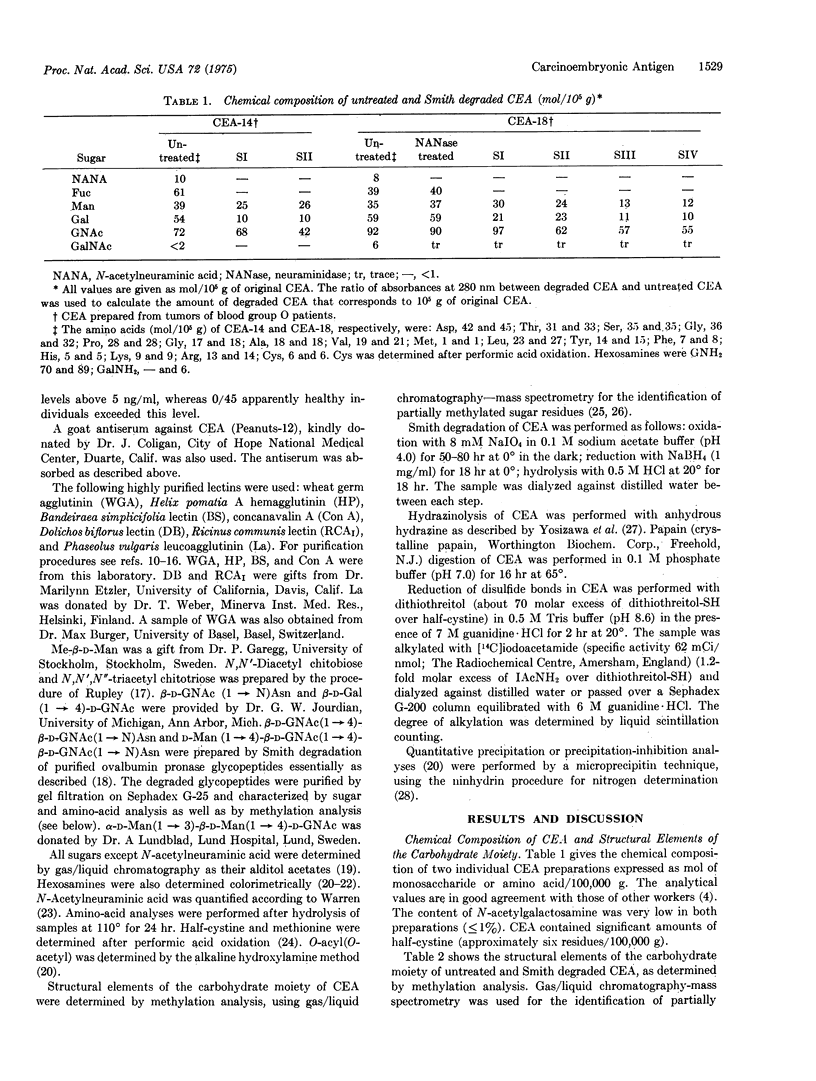
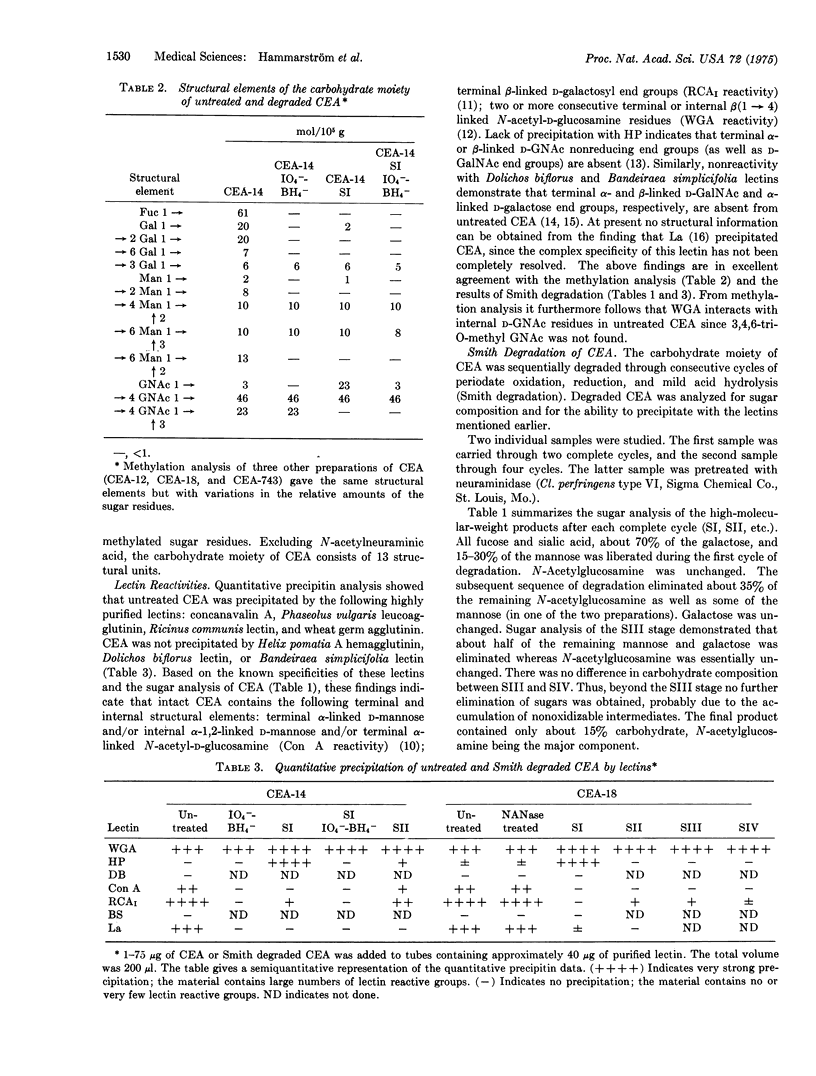
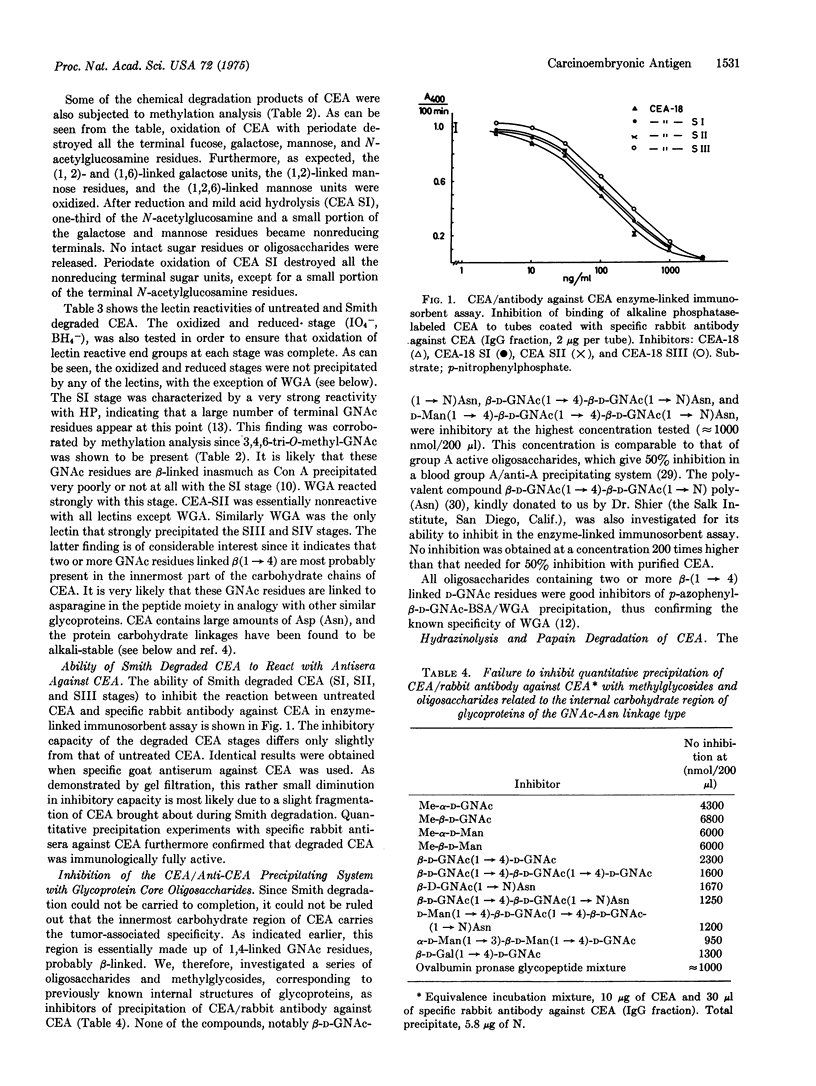
Abstract
The carbohydrate moiety of carcinoembryonic antigen could be sequentially degraded by repeated cycles of periodate oxidation, reduction, and mild acid hydrolysis (Smith degradation). After three complete degradations, all fucose and sialic acid, 80% of the galactose, 65% of the mannose, and about 40% of the N-acetylglucosamine were eliminated without impairing the ability of degraded carcinoembryonic antigen to react with specific antisera against the antigen. Inhibition studies in a carcinoembryonic antigen/rabbit anti-carcinoembryonic antigen precipitating system with oligosaccharides covering previously known internal structures of glycoproteins and presumably corresponding to the internal carbohydrate region of the antigen, demonstrated that none of the compounds tested was inhibitory. Nor could any inhibitory effect on the binding of carcinoembryonic antigen to antibody against the antigen in a radioimmunoassay system be domonstrated for the carbohydrate moiety prepared by hydrazinolysis or the glyco peptide fraction isolated after papain degradation of the antigen. However, if carcinoembryonic antigen is completely reduced and alkylated, with three intrachain disulfide bonds cleaved per 10-5 g, the immunological activity is reduced to 3-5% of untreated antigen. Furthermore, treatment of the antigen with 0.5 NaOH at 20 degrees for 2 hr completely abolished its ability to react with antiserum, whereas its ability to precipitate with a series of lectins was unchanged. No release of low-molecular-weight carbohydrate orchange in sugar composition of alkali-treated antigen was observed. Our tentative conclusion is that the carbohydrate moiety of carcinoembryonic antigen does not contain the tumor-associated determinant(s).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjo C., Gold P., Freedman S. O., Krupey J. Immunologically active heterosaccharides of carcinoembryonic antigen of human digestive system. Nat New Biol. 1972 Aug 9;238(84):183–185. doi: 10.1038/newbio238183a0. [DOI] [PubMed] [Google Scholar]
- Banjo C., Gold P., Gehrke C. W., Freedman S. O., Krupey J. Preparation and isolation of immunologically active glycopeptides from carcinoembryonic antigen (CEA). Int J Cancer. 1974 Feb 15;13(2):151–163. doi: 10.1002/ijc.2910130202. [DOI] [PubMed] [Google Scholar]
- Egan M. L., Todd C. W. Carcinoembryonic antigen: synthesis by a continuous line of adenocarcinoma cells. J Natl Cancer Inst. 1972 Sep;49(3):887–889. [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Kabat E. A. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970 Feb 17;9(4):869–877. doi: 10.1021/bi00806a022. [DOI] [PubMed] [Google Scholar]
- GOLD P., FREEDMAN S. O. DEMONSTRATION OF TUMOR-SPECIFIC ANTIGENS IN HUMAN COLONIC CARCINOMATA BY IMMUNOLOGICAL TOLERANCE AND ABSORPTION TECHNIQUES. J Exp Med. 1965 Mar 1;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P., Freedman S. O. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965 Sep 1;122(3):467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Reichert C. M., Misaki A. Interaction of concanavalin A with model substrates. Ann N Y Acad Sci. 1974;234(0):283–296. doi: 10.1111/j.1749-6632.1974.tb53040.x. [DOI] [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Krupey J., Wilson T., Freedman S. O., Gold P. The preparation of purified carcinoembryonic antigen of the human digestive system from large quantities of tumor tissue. Immunochemistry. 1972 Jun;9(6):617–622. doi: 10.1016/0019-2791(72)90247-9. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Layug E. J., Gruezo F. Immunochemical studies on blood groups. XXXIV. Structures of some oligosaccharides produced by alkaline degradation of blood group A, B, and H substances. Biochemistry. 1966 May;5(5):1489–1501. doi: 10.1021/bi00869a007. [DOI] [PubMed] [Google Scholar]
- Ludowieg J., Benmaman J. D. Colorimetric differentiation of hexosamines. Anal Biochem. 1967 Apr;19(1):80–88. doi: 10.1016/0003-2697(67)90136-4. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Pusztaszeri G. Carcinoembryonic antigen (CEA): demonstration of a partial identity between CEA and a normal glycoprotein. Immunochemistry. 1972 Oct;9(10):1031–1034. doi: 10.1016/0019-2791(72)90113-9. [DOI] [PubMed] [Google Scholar]
- Makino M., Yamashina I. Periodate oxidation of glycopeptides from ovalbumin. J Biochem. 1966 Sep;60(3):262–267. doi: 10.1093/oxfordjournals.jbchem.a128432. [DOI] [PubMed] [Google Scholar]
- Moreno C., Kabat E. A. Studies on human antibodies. 8. Properties and association constants of human antibodies to blood group A substance purified with insoluble specific adsorbents and fractionally fluted with mono- and oligosaccharide. J Exp Med. 1969 May 1;129(5):871–896. doi: 10.1084/jem.129.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A. M., Laurence D. J. Report of the workshop on the carcinoembryonic antigen (cea): the present position and proposals for future investigation. Int J Cancer. 1974 Jul 15;14(1):1–1. doi: 10.1002/ijc.2910140102. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Mechanism of denaturation of haemoglobin by alkali. Nature. 1974 Feb 8;247(5440):341–344. doi: 10.1038/247341a0. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Preparation of a "chemical vaccine" against tumor progression. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2078–2082. doi: 10.1073/pnas.68.9.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry W. D., Henkart P. A., Coligan J. E., Todd C. W. Carcinoembryonic antigen: characterization and clinical applications. Transplant Rev. 1974;20(0):100–129. doi: 10.1111/j.1600-065x.1974.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Thomson D. M., Krupey J., Freedman S. O., Gold P. The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci U S A. 1969 Sep;64(1):161–167. doi: 10.1073/pnas.64.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber T. H., Aro H., Nordman C. T. Characterization of lymphocyte-stimulating blood cell-agglutinating glycoproteins from red kidney beans (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Mar 15;263(1):94–105. doi: 10.1016/0005-2795(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Yosizawa Z., Sato T., Schmid K. Hydrazinolysis of alpha-1-acid glycoprotein. Biochim Biophys Acta. 1966 Jun 29;121(2):417–420. doi: 10.1016/0304-4165(66)90134-6. [DOI] [PubMed] [Google Scholar]


