Abstract
AIM: To investigate the survival outcomes of secondary hepatectomy for recurrent colorectal liver metastases (CRLM).
METHODS: From October 1994 to December 2009, patients with CRLM who underwent surgical treatment with curative intent were investigated. Patients were divided into two groups: patients who underwent primary hepatectomy (Group 1) and those who underwent secondary hepatectomy for recurrent CRLM (Group 2).
RESULTS: Survival and prognostic factors were analyzed. A total of 461 patients were included: 406 patients in Group 1 and 55 patients in Group 2. After a median 39-mo (range, 3-195 mo) follow-up, there was a significant difference between Groups 1 and 2 in terms of disease-free survival (P = 0.029) although there was no significant difference in overall survival (P = 0.206). Secondary hepatectomy was less effective in patients with multiple recurrent CRLM than primary hepatectomy for initial CRLM (P = 0.008). Multiple CRLM and radiofrequency ablation therapy were poor prognostic factors of secondary hepatectomy in multivariate Cox regression analysis (P = 0.006, P = 0.004, respectively).
CONCLUSION: Secondary hepatectomy for single recurrent CRLM is as effective as primary surgical treatment for single recurrent CRLM. However, secondary hepatectomy for multiple recurrent CRLM is less effective than that for single recurrent CRLM.
Keywords: Colorectal neoplasm, Metastasis, Recurrence, Hepatectomy
Core tip: Secondary hepatectomy for single recurrent colorectal liver metastases (CRLM) is as effective as primary surgical treatment for single recurrent CRLM. However, secondary hepatectomy for multiple recurrent CRLM is less effective than that for single recurrent CRLM.
INTRODUCTION
Hepatic resection is the standard treatment for colorectal liver metastases (CRLM). The 5-year overall survival (OS) rate has been estimated to be as high as 58%[1]. Although initial hepatectomy for CRLM is potentially curative, repeat hepatectomy has been reported to have relatively limited value[2].
Many studies have evaluated the outcomes of repeat hepatectomy; repeat hepatectomy has been found to be a feasible treatment option for recurrent CRLM. The 5-year OS rate was estimated to be as high as 54% in patients who underwent repeat hepatectomy for recurrent CRLM[2-9]. Number, size, location of lesions, metachronous CRLM, high carcinoembryonic antigen (CEA) levels, and extrahepatic metastasis have been reported to be risk factors of poor prognosis after repeat hepatectomy for recurrent CRLM.
However, few studies have compared survival curves between primary and secondary hepatectomy with long-term follow-up data. Moreover, it is unknown whether repeat hepatectomy is reasonable in patients with poor prognostic factors. The aim of this study was to investigate survival outcomes of secondary hepatectomy for recurrent CRLM compared with primary hepatectomy. We also evaluated the outcomes of secondary hepatectomy in patients with poor prognostic factors.
MATERIALS AND METHODS
From September 1994 to December 2009, colorectal cancer patients with synchronous or metachronous CRLM who underwent curative intent surgical treatment were identified from a prospectively collected database. Extrahepatic metastasis, double primary carcinoma, grossly remnant tumor after surgery, hereditary nonpolyposis colorectal cancer, and familial adenomatous polyposis were exclusion criteria.
Patients were divided into two groups. Patients who underwent initial surgical treatment for CRLM were assigned to Group 1, while those who underwent surgical treatment for repeat CRLM were assigned to Group 2. In Group 1, the patients who underwent repeat hepatectomy during follow-up periods were excluded.
Patients underwent surgical treatment for primary colorectal cancer and CRLM if the CRLM was considered surgically curable. Results of surgical treatment were evaluated according to American Society of Clinical Oncology (AJCC) criteria: no residual tumor left after resection (R0), microscopic tumor remains (R1), or margins involved or gross disease remains (R2).
Surgical treatment for CRLM included hepatic resection and radiofrequency ablation (RFA). RFA was performed using open surgical, laparoscopic, or percutaneous approaches by interventional radiologists. An expendable needle radiofrequency system (460 KHz generator model 500 or 1500; RITA Medical Systems, Mountain View, CA, cool tip, Radionics Corporation, Burlington, MA) using single or clustered tip was used. In all patients who underwent RFA, complete necrosis of liver metastasis was confirmed by intraoperative ultrasonography and a postoperative computed tomography scan within 1 wk of the procedure.
Patients were treated with adjuvant chemotherapy based on fluorouracil. Neoadjuvant combined chemotherapy and radiation therapy was mainly performed in rectal cancer patients to facilitate sphincter preservation.
Postoperative surveillance for recurrence was performed every 3 to 6 mo for 3 years and annually thereafter; this included physical examination, chest x-ray, and abdominal computed tomography scanning. Roentgen images in addition to medical records were reviewed retrospectively to determine recurrence. Endpoints of this study were the time to tumor recurrence and time to death.
Sex, age, number and size of hepatic metastases, T stage, N stage, cell differentiation, lymphatic invasion, vascular invasion, perineural invasion of primary colorectal cancer, chemotherapy, recurrences, death, disease-free survival, and OS were the variables investigated in each patient. T and N stage of colorectal cancer were determined according to the seventh edition of the AJCC[10].
Categorical variables are reported as numbers (percentages). Continuous variables are reported as medians (ranges). Categorical variables were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U test. Survival was calculated using the Kaplan-Meier method from the date of surgical treatment, and differences in survival were examined using the log-rank test. Risk factors were analyzed using a Cox regression model. Cox proportional hazards regression was used to assess the individual contribution of factors associated with survival. All variables that were significant in univariate analysis were verified with a multivariate model.
P values less than 0.05 were considered statistically significant.
RESULTS
Among 10189 colorectal cancer patients, 461 patients who were diagnosed with CRLM and underwent R0 surgical treatment were identified. There were 281 recurrences after initial hepatectomy, and 55 patients who underwent R0 secondary hepatectomy were identified. Patients with exclusion criteria were excluded. Finally, 406 patients of Group 1 and 55 patients of Group 2 were identified and analyzed.
Median age was 59 years (range, 26-80 years) and median follow-up period was 39 mo (range, 3-195 mo) (Figure 1).
Figure 1.
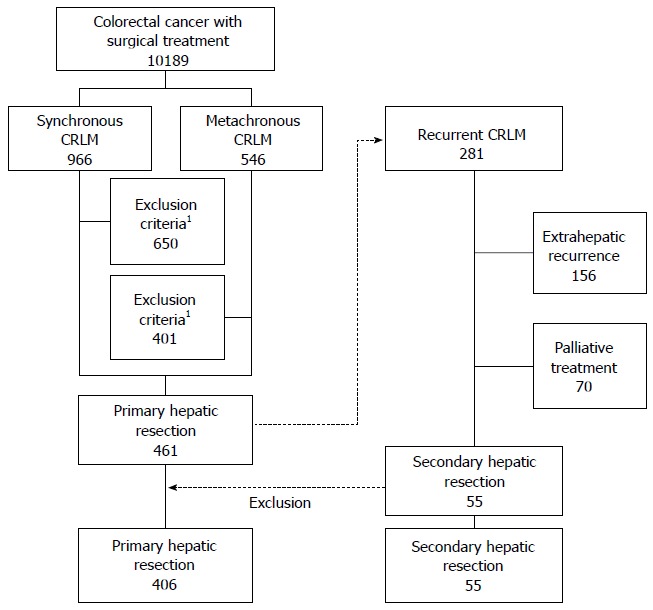
Flow diagram of patient selection. 1Exclusion criteria: extrahepatic metastasis, double primary carcinoma, grossly remnant tumor after surgery, hereditary nonpolyposis colorectal cancer, and familial adenomatous polyposis.
Demographics
Sex, age, size of CRLM, adjuvant chemotherapy, T stage, N stage, lymphatic invasion, vascular invasion, perineural invasion, and histological differentiation of primary colorectal cancer were not significantly different between the two groups. The number of CRLM and the RFA proportion were significantly different between the two groups (P = 0.006 and P < 0.001, respectively) (Table 1).
Table 1.
Characteristics of patients with colorectal liver metastases n (%)
| Variable | Primary hepatectomy (n = 406) | Secondary hepatectomy (n = 55) | P value1 |
| Sex (M/F) | 266:140 | 36:19 | 0.99 |
| Median age (yr) (range) | 59.5 (26-80) | 58 (36-80) | 0.95 |
| Number of CRLM (median, range) | 1 (1-8) | 1 (1-4) | 0.01 |
| CRLM size (median, range) | 2.4 (0.2-7.5) | 2.4 (1.0-7.5) | 0.85 |
| Liver metastases | |||
| Synchronous | 279 (69) | 39 (71) | 0.74 |
| Metachronous | 127 (31) | 16 (29) | |
| CRLM number | |||
| Single | 232 (57) | 41 (75) | 0.014 |
| Multiple | 174 (43) | 14 (25) | |
| CRLM treatment | |||
| Resection | 324 (80) | 25 (45) | < 0.001 |
| RFA | 57 (14) | 29 (53) | |
| Both | 25 (6) | 1 (2) | |
| T stage | |||
| T1 T2 T3 | 364 (90) | 47 (85) | 0.35 |
| T4 | 42 (10) | 8 (15) | |
| N stage | |||
| N0 | 117 (29) | 13 (24) | 0.42 |
| N1 or N2 | 289 (71) | 42 (76) | |
| Histological differentiation2 | |||
| High grade | 367 (90) | 53 (96) | 0.21 |
| Low grade | 39 (10) | 2 (4) | |
| Lymphatic invasion | |||
| (-) | 172 (42) | 16 (29) | 0.02 |
| (+) | 129 (32) | 26 (47) | |
| Vascular invasion | |||
| (-) | 168 (41) | 29 (53) | 0.14 |
| (+) | 102 (25) | 10 (18) | |
| Perineural invasion | |||
| (-) | 191 (47) | 20 (36) | 0.38 |
| (+) | 74 (18) | 11 (22) | |
| Chemotherapy | |||
| Yes | 373 (92) | 50 (91) | 0.79 |
| No | 33 (8) | 5 (9) |
χ2 test, Fisher’s exact test, or Mann Whitney U test;
High grade: well or moderately differentiated, Low grade: poorly differentiated or mucinous carcinoma. CRLM: Colorectal liver metastases.
Perioperative outcomes
The morbidity rate was 17% in Group 1 and 11% in Group 2 (P = 0.23). There were no statistically significant differences in complication rate or hospital stay between the two groups. Mean operation time of the initial hepatectomy was 276.4 min (SD ± 101.3). Mean operation time of the secondary hepatectomy was 263.5 min (SD ± 58.7). Initial operation time was longer than that of the secondary hepatectomy because it included the surgery time to remove the primary colorectal cancer.
Poor prognostic factors
Prognostic factors were evaluated using the Cox regression test in patients who underwent repeat hepatectomy. Size of CRLM, histologic low grade cell differentiation and lymphatic invasion of primary colorectal cancer were significant prognostic factors for DFS in the primary hepatectomy group (P = 0.006, P = 0.001 and P = 0.040, respectively). Multiplicity of CRLM and RFA were significant prognostic factors for DFS in the secondary hepatectomy group (P = 0.006 and P = 0.004, respectively) (Table 2).
Table 2.
Cox regression proportional hazard model of disease-free survival in recurrent colorectal liver metastases
| Variable |
Primary hepatectomy (n = 406) |
Secondary hepatectomy (n = 55) |
||||||
| Univariate analysis |
Multivariate analysis1 |
Univariate analysis |
Multivariate analysis1 |
|||||
| P value | HR | 95%CI | P value | P value | HR | 95%CI | P value | |
| Male sex | 0.110 | 0.260 | ||||||
| Age | 0.750 | 0.360 | ||||||
| Metachronous | 0.002 | 0.70 | 0.42-1.16 | 0.170 | 0.670 | |||
| Multiple CRLM | 0.067 | 0.037 | 2.78 | 1.33-5.78 | 0.006 | |||
| Size of CRLM | 0.001 | 1.15 | 1.04-1.26 | 0.006 | 0.260 | |||
| Radiofrequency ablation | 0.220 | 0.008 | 2.84 | 1.40-5.73 | 0.004 | |||
| T4 | 0.110 | 0.530 | ||||||
| N1 or N2 | 0.002 | 1.17 | 0.70-1.94 | 0.550 | 0.310 | |||
| Low grade cell differentiation2 | < 0.001 | 2.38 | 1.46-3.88 | 0.001 | 0.660 | |||
| Lymphatic invasion | < 0.001 | 1.50 | 1.02-2.20 | 0.040 | 0.100 | |||
| Vascular invasion | < 0.001 | 1.49 | 0.99-2.25 | 0.058 | 0.670 | |||
| Perineural invasion | 0.001 | 1.32 | 0.88-1.99 | 0.190 | 0.820 | |||
| Adjuvant chemotherapy | 0.950 | 0.140 | ||||||
Variables that were significant in univariate analysis were verified with a multivariate model;
Low grade: poorly differentiated or mucinous carcinoma. CRLM: Colorectal liver metastases.
Survival
DFS and OS curves of the initial hepatic resection group appeared superior to those of the secondary hepatectomy group. There was a significant difference in terms of DFS (P = 0.029) although there was no significant difference in OS (P = 0.206) (Figure 2).
Figure 2.
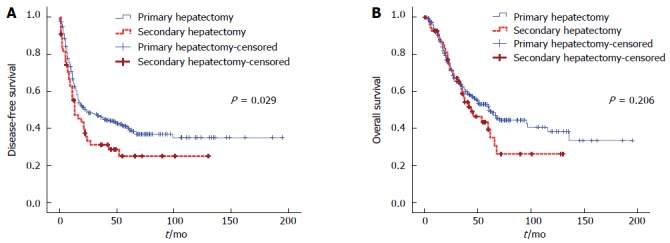
Disease-free survival and overall survival curves after primary and secondary hepatic resection for colorectal liver metastases. A: Disease-free survival; B: Overall survival.
One, 3-, and 5-year DFS rates were 66.9%, 46.6%, and 40.3%, respectively, in the primary hepatectomy group vs 55.2%, 31.2%, and 25.0%, respectively, in the secondary hepatectomy group. One, 3-, and 5-year OS rates were 91.9%, 63.3%, and 53.0%, respectively, in the primary hepatectomy group compared to 92.6%, 58.7%, and 43.3%, respectively, in the secondary hepatectomy group.
DFS curves were analyzed according to prognostic factors associated with secondary CRLM. Survival curves of the primary and secondary hepatectomy groups differed according to the number of CRLM. Differences were very obvious in the secondary hepatectomy group (P = 0.029). Median DFS time was 17 mo after primary hepatectomy and 7 mo after secondary hepatectomy for multiple CRLM. Secondary hepatectomy for cases of multiple recurrent CRLM was not more effective than primary hepatectomy for initial CRLM (P = 0.008) (Figure 3).
Figure 3.
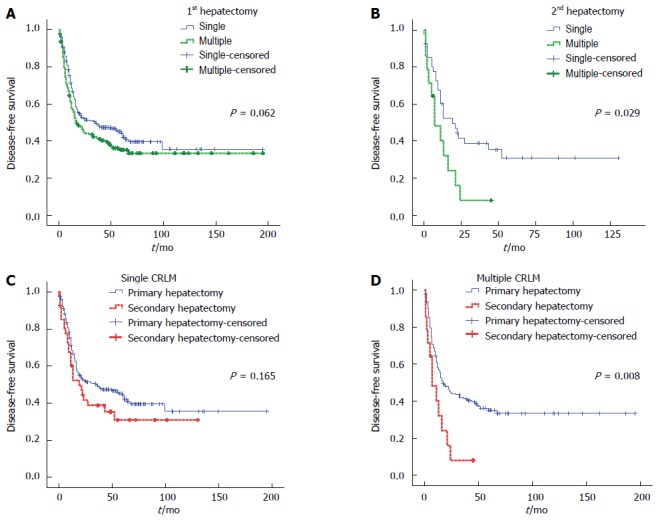
Disease-free survival curves after primary and secondary hepatic resection for single or multiple colorectal liver metastases. A: Primary hepatectomy for single or multiple colorectal liver metastases (CRLM); B: Secondary hepatectomy for single or multiple recurrent CRLM; C: Primary or secondary hepatectomy for single CRLM; D: Primary or secondary hepatectomy for multiple CRLM.
DISCUSSION
Survival outcomes were similar between patients undergoing primary or secondary hepatectomy for single CRLM in this study. However, surgical treatment was less effective for multiple CRLM. Though patients underwent secondary hepatectomy, the recurrence rate was high if there were multiple CRLM. In previous studies, the number of CRLM was thought to be a prognostic factor for repeat hepatectomy. However, this has not been well studied compared with primary hepatectomy[6,11].
Major hepatectomy is associated with high morbidity rate. Recent studies have reported the morbidity rate to still be up to 60% after hepatectomy for CRLM[12]. However, repeat hepatectomy could be justified with acceptable morbidity and mortality compared with primary hepatectomy. Many studies reported that repeat hepatectomy for recurrent CRLM is safe enough and improves survival outcomes[13]. In our study, the morbidity rate was 11% after secondary hepatectomy and lower than in the primary hepatectomy group. However, the primary hepatectomy group included patients with synchronous CRLM who underwent colorectal surgery and hepatectomy simultaneously. Therefore, the morbidity rate was not exactly compared between the two groups. We expect the morbidity rate to seem similar if the proportion of colorectal surgery is excluded.
In this study, the 5-year survival rate was more than 40% after secondary hepatectomy for recurrent CRLM. Although excellent overall survival outcomes have been observed in patients who underwent repeat hepatectomy, the high recurrence rate remains an unsolved problem[14]. The recurrence rate was significantly higher in the secondary hepatectomy group then in the primary hepatectomy group. Half of the patients experienced recurrence after secondary hepatectomy. If there were multiple recurrent CRLM, median DFS time was only 7 mo. Short-term follow-up is necessary after repeat hepatectomy, especially for multiple recurrent CRLM. To detect recurrent CRLM effectively, various markers have been investigated[15]. However, molecular markers have not been well established yet.
In the study, RFA was a significant risk factor of recurrence. According to a recent study, cluster of differentiation 95 (CD95) is thought to play a role in the recurrence of cancer after RFA because of the potential of RFA to cause hypoxic damage. CD95 can induce apoptosis, but can also promote tumor genesis in apoptosis-resistant tumor cells[16]. However, we expect there might be a selection bias. Hepatectomy is the most effective curative treatment for resectable metastatic liver disease, including recurrent metastases. We consider hepatectomy to be the first-line treatment for CRLM. We inevitably choose RFA as a substitute for hepatectomy when there are multiple CRLM and expected remnant liver volume is too small. The patients who have recurrent CRLM are older than initial CRLM patients and the risks of morbidity increase with age. If the patients have severe comorbidities, RFA might be safer than hepatectomy. Therefore, we could not evaluate the efficiency of RFA compared with hepatectomy.
There are still controversies regarding the indications for repeat hepatectomy. A recent study reported that hepatectomy for an old-age group is feasible with a reasonable long term survival rate[17]. Repeat hepatectomy was also attempted for recurrent gastric cancer and it was reported that it could offer the chance for cure in selected patients[18]. However, hepatectomy is not always possible, especially in recurrent CRLM. In patients with unresectable recurrent CRLM, liver transplantation was not indicated in the past. However, a recent study reported that the oncologic outcomes of liver transplantation for unresectable CRLM is comparable with liver resection for resectable CRLM[19].
In our study, we reviewed the age of patients according to the date of hepatectomy. To reduce bias, we also performed multivariate analysis. However, there may have been selection bias. There was a difference in CRLM number between the two groups. In the secondary hepatectomy group, single CRLM was more common than in the primary hepatectomy group. However, this study has an adequate number of patients and long-term follow-up data. Moreover, a randomized controlled study design is impossible for repeat hepatic resection. Although there are limitations in this study, we were able to find significant prognostic factors of secondary hepatectomy.
Previous studies have reported that repeat hepatectomy is safe and effective for recurrent CRLM[20,21]. However, surgeons should be cautious before performing surgery in patients with poor prognostic factors. Secondary hepatectomy was not effective for multiple recurrent CRLM and the median DFS time was only 7 mo. In those patients, detailed preoperative evaluation is compulsory and short-term follow-up is necessary after secondary hepatectomy.
In conclusion, secondary hepatectomy for single recurrent CRLM is as effective as primary surgical treatment for single CRLM. However, secondary hepatectomy for multiple recurrent CRLM is less effective than that for single recurrent CRLM.
COMMENTS
Background
Hepatic resection is the standard treatment for colorectal liver metastases (CRLM). Although initial hepatectomy for CRLM is potentially curative, repeat hepatectomy has been reported to have relatively limited value. Only a few studies have compared survival curves between primary and secondary hepatectomy. It is unclear whether repeat hepatectomy is as safe and effective as primary hepatectomy, especially in patients with poor prognostic factors.
Research frontiers
Secondary hepatectomy for recurrent CRLM is as effective as primary surgical treatment for CRLM. However, high recurrence rates still remain an unsolved problem. The research hotspot is how to reduce recurrence rates after repeat hepatectomy. Many chemotherapeutic drugs including targeted therapy agents have been developed. However, the optimal treatment for recurrent CRLM has not been established yet.
Innovations and breakthroughs
The authors found that the prognostic factors are different between primary hepatectomy group and secondary hepatectomy groups. The recurrence rate increased according to multiplicity of CRLM in the secondary hepatectomy group. However, it was not a significant prognostic factor for primary hepatectomy for CRLM.
Applications
The study results suggest that secondary hepatectomy for single recurrent CRLM is as effective as primary surgical treatment for single CRLM. However, secondary hepatectomy for multiple recurrent CRLM is less effective than that for single recurrent CRLM. They think that the above findings and our conclusion are quite new and can help clinicians determine what they should do when they encounter patients with recurrent CRLM.
Peer-review
This is a good descriptive study in which the authors analyzed the survival outcomes according to repeat hepatectomy and risk factors. The results are clear and suggest the recommendations of secondary hepatectomy.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 14, 2014
First decision: August 15, 2014
Article in press: September 30, 2014
P- Reviewer: Balaban YH, Moralioglu S, Nigri G S- Editor: Qi Y L- Editor: Logan S E- Editor: Zhang DN
References
- 1.Haddad AJ, Bani Hani M, Pawlik TM, Cunningham SC. Colorectal liver metastases. Int J Surg Oncol. 2011;2011:285840. doi: 10.1155/2011/285840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones NB, McNally ME, Malhotra L, Abdel-Misih S, Martin EW, Bloomston M, Schmidt CR. Repeat hepatectomy for metastatic colorectal cancer is safe but marginally effective. Ann Surg Oncol. 2012;19:2224–2229. doi: 10.1245/s10434-011-2179-0. [DOI] [PubMed] [Google Scholar]
- 3.Lopez P, Marzano E, Piardi T, Pessaux P. Repeat hepatectomy for liver metastases from colorectal primary cancer: a review of the literature. J Visc Surg. 2012;149:e97–e103. doi: 10.1016/j.jviscsurg.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Wicherts DA, de Haas RJ, Salloum C, Andreani P, Pascal G, Sotirov D, Adam R, Castaing D, Azoulay D. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100:808–818. doi: 10.1002/bjs.9088. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, Sim J, Black D, Niu R, Morris DL. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2069–2077. doi: 10.1245/s10434-007-9388-6. [DOI] [PubMed] [Google Scholar]
- 6.Adair RA, Young AL, Cockbain AJ, Malde D, Prasad KR, Lodge JP, Toogood GJ. Repeat hepatic resection for colorectal liver metastases. Br J Surg. 2012;99:1278–1283. doi: 10.1002/bjs.8845. [DOI] [PubMed] [Google Scholar]
- 7.Brachet D, Lermite E, Rouquette A, Lorimier G, Hamy A, Arnaud JP. Prognostic factors of survival in repeat liver resection for recurrent colorectal metastases: review of sixty-two cases treated at a single institution. Dis Colon Rectum. 2009;52:475–483. doi: 10.1007/DCR.0b013e31819d12bc. [DOI] [PubMed] [Google Scholar]
- 8.Battula N, Tsapralis D, Mayer D, Isaac J, Muiesan P, Sutcliffe RP, Bramhall S, Mirza D, Marudanayagam R. Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB (Oxford) 2014;16:157–163. doi: 10.1111/hpb.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziff O, Rajput I, Adair R, Toogood GJ, Prasad KR, Lodge JP. Repeat liver resection after a hepatic or extended hepatic trisectionectomy for colorectal liver metastasis. HPB (Oxford) 2014;16:212–219. doi: 10.1111/hpb.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 11.Kulik U, Bektas H, Klempnauer J, Lehner F. Repeat liver resection for colorectal metastases. Br J Surg. 2013;100:926–932. doi: 10.1002/bjs.9132. [DOI] [PubMed] [Google Scholar]
- 12.Wei M, He Y, Wang J, Chen N, Zhou Z, Wang Z. Laparoscopic versus open hepatectomy with or without synchronous colectomy for colorectal liver metastasis: a meta-analysis. PLoS One. 2014;9:e87461. doi: 10.1371/journal.pone.0087461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda A, Matsumoto S, Seya T, Matsutani T, Kishi T, Yokoi K, Wang P, Uchida E. Does postoperative complication have a negative impact on long-term outcomes following hepatic resection for colorectal liver metastasis?: a meta-analysis. Ann Surg Oncol. 2013;20:2485–2492. doi: 10.1245/s10434-013-2972-z. [DOI] [PubMed] [Google Scholar]
- 14.Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 2011;13:774–782. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassmann S, Tang L, Capanu M, Brabletz T, Schöpflin A, Zur Hausen A, Gonen M, Kemeny N, Shia J, Klimstra D, et al. Predictive molecular markers for colorectal cancer patients with resected liver metastasis and adjuvant chemotherapy. Gastroenterology. 2007;133:1831–1839. doi: 10.1053/j.gastro.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 16.Nijkamp MW, Hoogwater FJ, Steller EJ, Westendorp BF, van der Meulen TA, Leenders MW, Borel Rinkes IH, Kranenburg O. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol. 2010;53:1069–1077. doi: 10.1016/j.jhep.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Kulik U, Framke T, Grosshennig A, Ceylan A, Bektas H, Klempnauer J, Lehner F. Liver resection of colorectal liver metastases in elderly patients. World J Surg. 2011;35:2063–2072. doi: 10.1007/s00268-011-1180-x. [DOI] [PubMed] [Google Scholar]
- 18.Takemura N, Saiura A, Koga R, Yoshioka R, Yamamoto J, Kokudo N. Repeat hepatectomy for recurrent liver metastasis from gastric carcinoma. World J Surg. 2013;37:2664–2670. doi: 10.1007/s00268-013-2190-7. [DOI] [PubMed] [Google Scholar]
- 19.Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, Fosby B, Boberg KM, Mathisen O, Gladhaug IP, Egge TS, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257:800–806. doi: 10.1097/SLA.0b013e3182823957. [DOI] [PubMed] [Google Scholar]
- 20.Thelen A, Jonas S, Benckert C, Schumacher G, Lopez-Hänninen E, Rudolph B, Neumann U, Neuhaus P. Repeat liver resection for recurrent liver metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:324–328. doi: 10.1016/j.ejso.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Chok KS, Cheung TT, Chan AC, Dai WC, Chan SC, Fan ST, Poon RT, Lo CM. Survival outcome of re-resection for recurrent liver metastases of colorectal cancer: a retrospective study. ANZ J Surg. 2014;84:545–549. doi: 10.1111/ans.12298. [DOI] [PubMed] [Google Scholar]


