Abstract
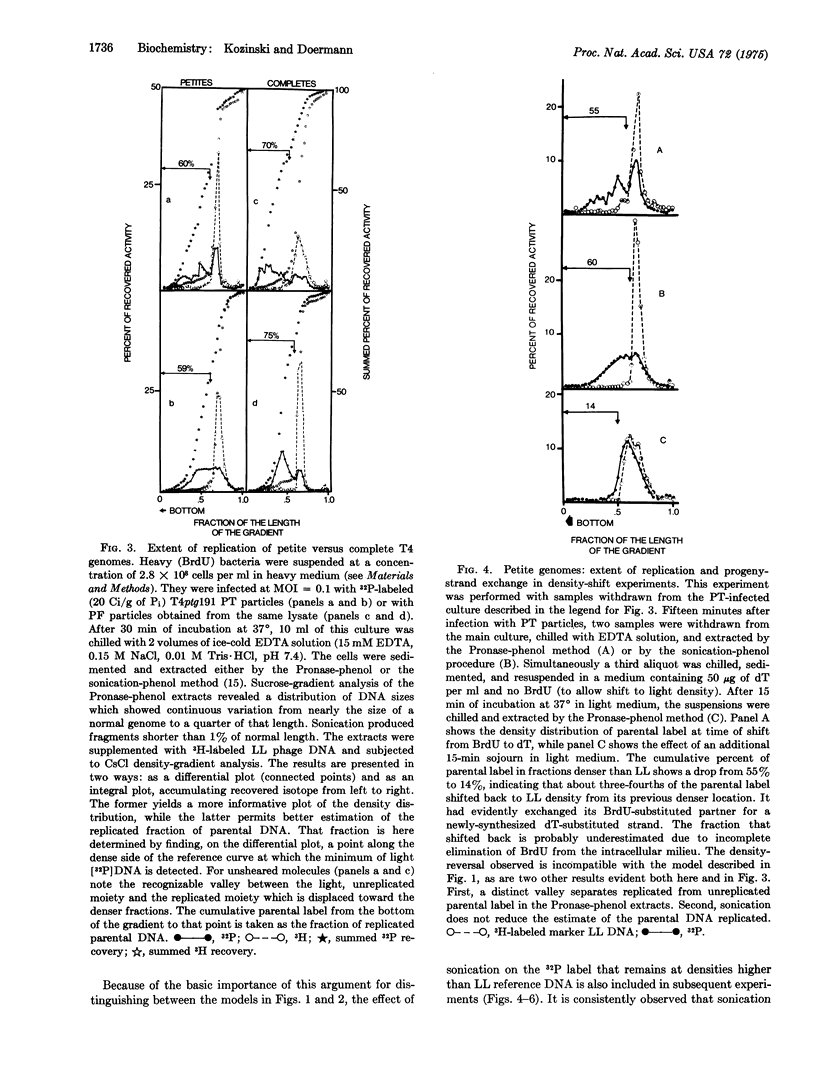
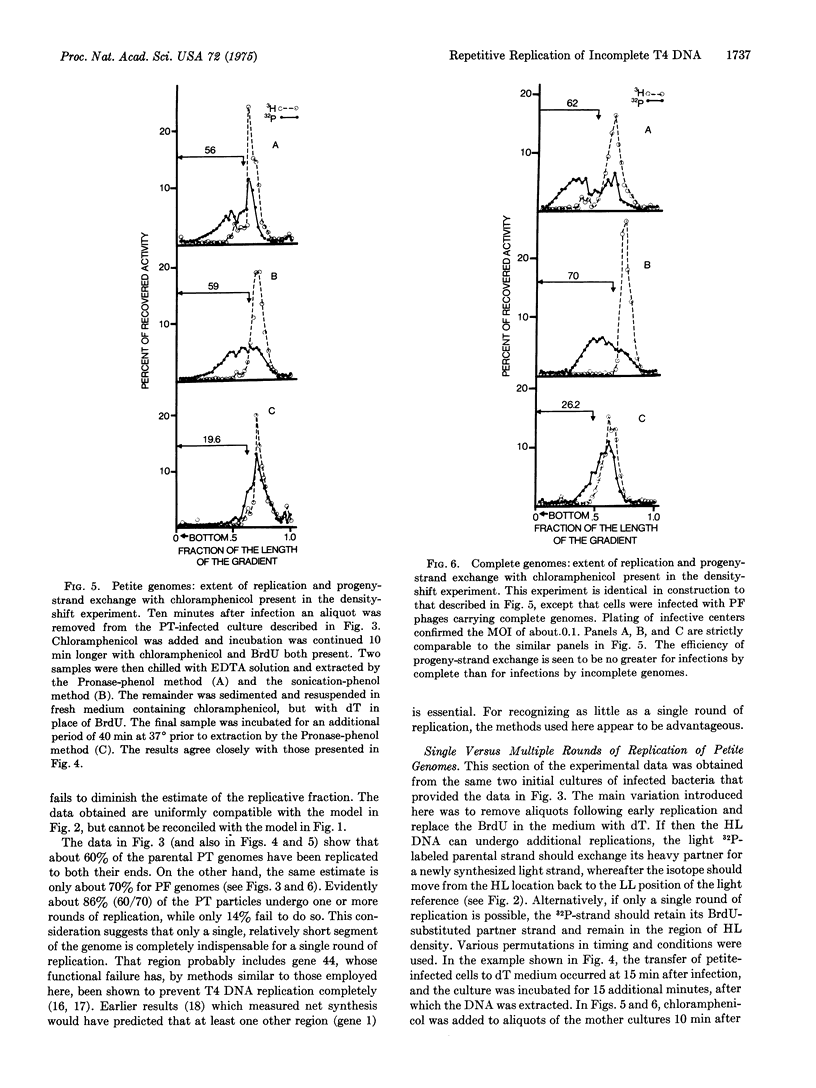
The genomes of petite T4 phage particles presumably cannot circularize because they are deficient for a significant terminal segment and hence not terminally redundant like normal T4 genomes. Combined density- and 32P-labeling shows that the majority of such deficient DNA molecules can nevertheless replicate their entire length. Furthermore, the density-shift technique shows that replicated parental strands can exchange their partners for new light strands, indicating that noncircularized T4 DNA molecules replicate repeatedly. When taken together with previously published data, these results indicate that T4 replication is bidirectional from multiple, genetically fixed points of origin. Rolling circle models can, therefore, not be considered as an essential mechanism for the early rounds of T4 replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Childs J. D. Permuted partial phage within single bursts of Escherichia coli infected with a T4D mutant. Genetics. 1969 Oct;63(2):239–246. doi: 10.1093/genetics/63.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H., Eiserling F. A., Boehner L. Genetic control of capsid length in bacteriophage T4. I. Isolation and preliminary description of four new mutants. J Virol. 1973 Aug;12(2):374–385. doi: 10.1128/jvi.12.2.374-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H. T4 and the rolling circle model of replication. Annu Rev Genet. 1973;7:325–341. doi: 10.1146/annurev.ge.07.120173.001545. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. C., Breschkin A. M., Mosig G. Origin and direction of bacteriophage T4 DNA replication. II. A gradient of marker frequencies in partially replicated T4 DNA as assayed by transformation. J Mol Biol. 1971 Sep 14;60(2):213–233. doi: 10.1016/0022-2836(71)90289-0. [DOI] [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. A preferred origin and direction of bacteriophage T4 DNA replication. I. A gradient of allele frequencies in crosses between normal and small T4 particles. J Mol Biol. 1970 Nov 14;53(3):503–514. doi: 10.1016/0022-2836(70)90080-x. [DOI] [PubMed] [Google Scholar]
- Mosig G. Distances separating genetic markers in T4 DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H. The structure of genomes of individual petit particles of the bacteriophage T4D mutant E920/96/41. Genetics. 1969 Oct;63(2):247–261. doi: 10.1093/genetics/63.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Bi-directional initiation of DNA synthesis in developing chick erythroblasts. Nat New Biol. 1972 Apr 19;236(68):195–197. doi: 10.1038/newbio236195a0. [DOI] [PubMed] [Google Scholar]


