Abstract
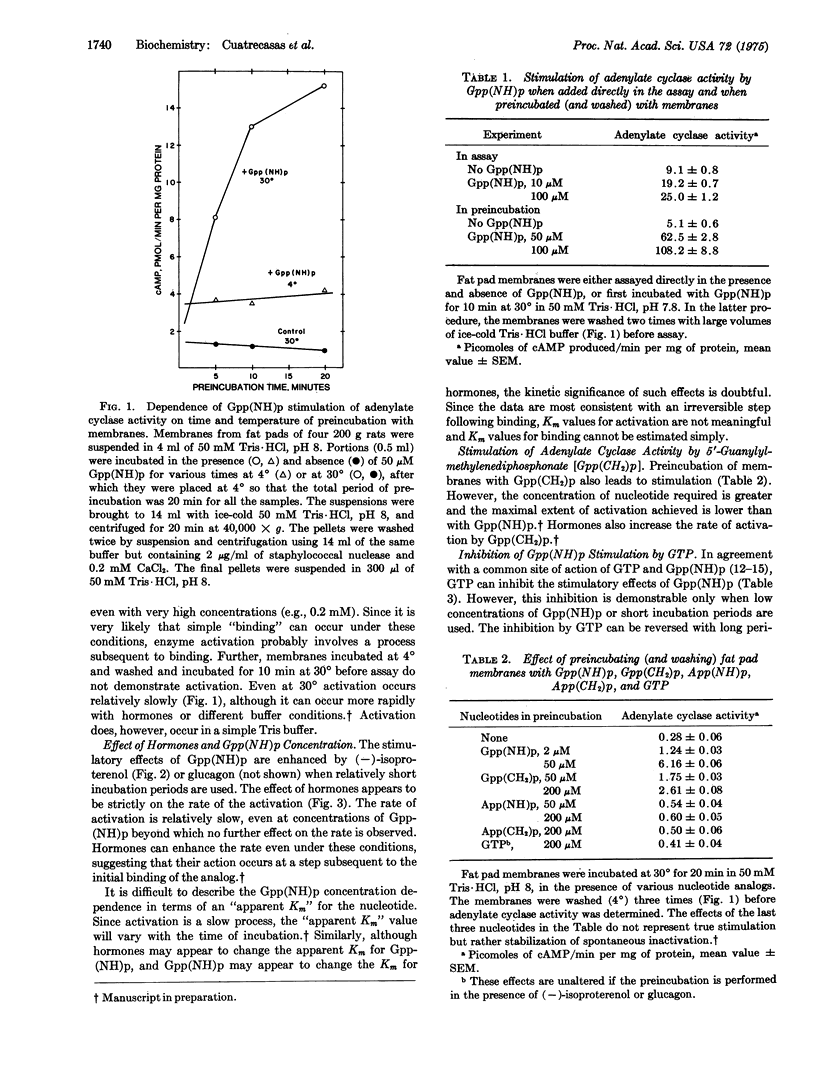
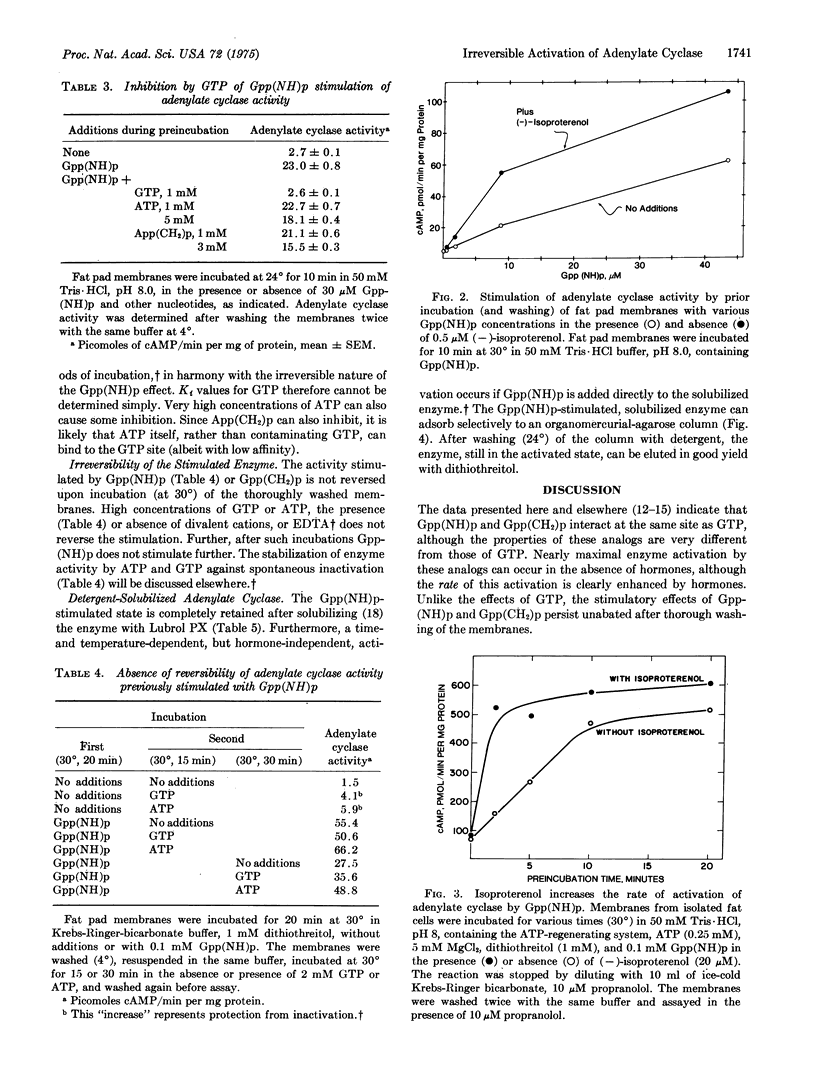
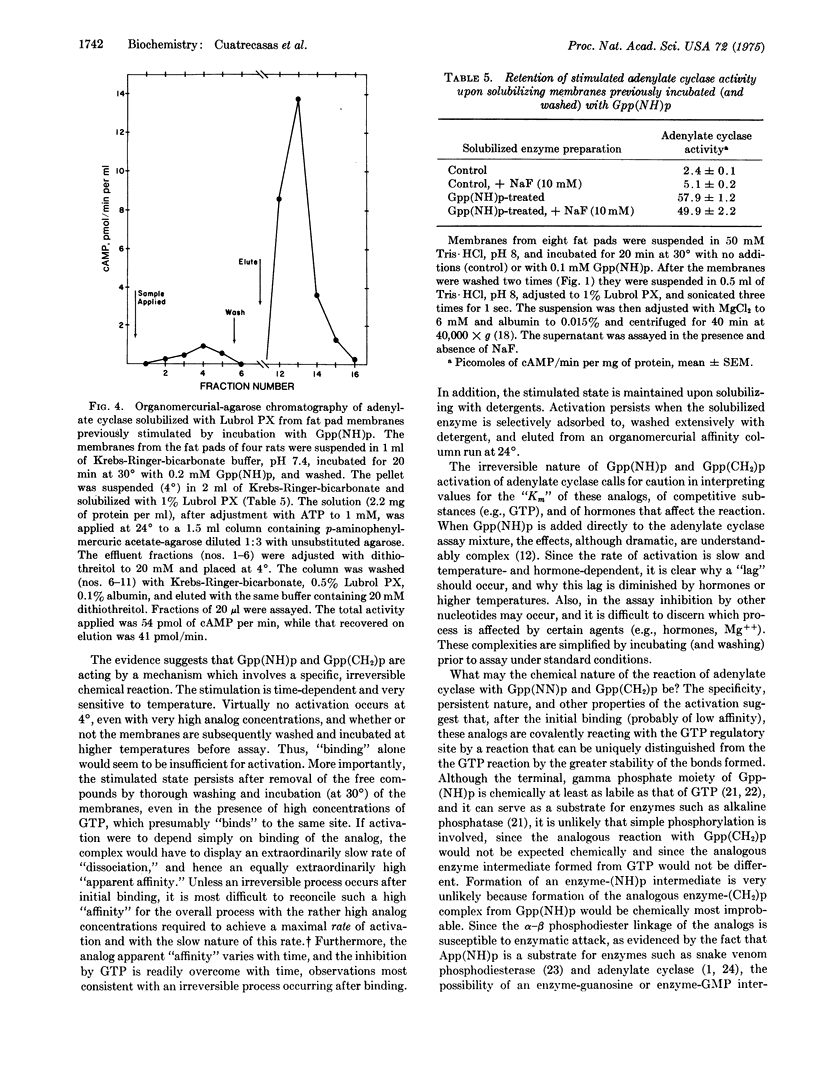
Incubation of rat fat pad membranes with 5-guanylyliminodiphosphonate [Gpp-(NH)p] and 5-guanylylmethylenediphosphonate [Gpp(CH2)p], but not GTP (with or without hormones), at 24 degrees or 30 degrees (but not at 4 degrees) greatly stimulates adenylate cyclase activity [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] measured after thoroughly washing the membranes. The rate of activation is relatively slow, even with very high (and saturating) concentrations of the analogs. Binding alone appears to be insufficient for activation. Hormones (catecholamines, glucagon) increase the rate but not the extent of activation, even when saturating analog concentrations are used. The dependence on analog concentration (apparent Km) varies with the time of incubation. GTP and very high concentrations of ATP inhibit the activation by Gpp(NH)p, but this effect is dependent on the length of incubation and can be overcome with time. The activated state is not reversed upon incubation of the washed membranes at 30 degrees, even in the presence of GTP, or by solubilization with nonionic detergents. Also, Gpp(NH)p can directly stimulate the control, solubilized enzyme. The activated state of the solubilized enzyme persists upon specific adsorption to and subsequent elution from an organomercurial-agarose column. It is suggested that after forming reversible Michaelis complexes of relatively low affinity, these analogs may react irreversibly with the GTP regulatory site of the enzyme, perhaps forming p(NH)p- and p(CH2)p-covalent enzyme intermediates which capture the activated state of the enzyme. GTP, after binding, may normally activate the enzyme by forming a "labile" pyrophosphoryl enzyme intermediate, and hormone receptors may function to increase the rate of formation (and thus concentration) of this active state of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., O'Keefe E., Cuatrecasaş P. Mechanism of action of cholera toxin and the mobile receptor theory of hormone receptor-adenylate cyclase interactions. Proc Natl Acad Sci U S A. 1975 Jan;72(1):33–37. doi: 10.1073/pnas.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J. P., Aurbach G. D. The effects of nucleotides on the expression of beta-adrenergic adenylate cyclase activity in membranes from turkey erythrocytes. J Biol Chem. 1974 Jan 10;249(1):157–161. [PubMed] [Google Scholar]
- Birnbaumer L., Nakahara T., Yang P. C. Studies on receptor-mediated activation of adenylyl cyclases. II. Nucleotide and nucleoside regulation of the activities of the beef renal medullary adenylyl cyclase and their stimulation by neurohypophyseal hormones. J Biol Chem. 1974 Dec 25;249(24):7857–7866. [PubMed] [Google Scholar]
- Bockaert J., Roy C., Jard S. Oxytocin-sensitive adenylate cyclase in frog bladder epithelial cells. Role of calcium, nucleotides, and other factors in hormonal stimulation. J Biol Chem. 1972 Nov 10;247(21):7073–7081. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Deery D. J., Howell S. L. Rat anterior pituitary adenyl cyclase activity. GTP requirement of prostaglandin E1 and E2 and synthetic luteinising hormone-releasing hormone activation. Biochim Biophys Acta. 1973 Nov 2;329(1):17–22. doi: 10.1016/0304-4165(73)90004-4. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Löw H., Rodbell M. Stimulatory and inhibitory effects of guanyl nucleotides on fat cell adenylate cyclase. J Biol Chem. 1973 Sep 10;248(17):6239–6245. [PubMed] [Google Scholar]
- Johnson D. G., Thompson W. J., Williams R. H. Regulation of adenylyl cyclase from isolated pancretic islets by prostaglandins and guanosine 5'-triphosphate. Biochemistry. 1974 Apr 23;13(9):1920–1924. doi: 10.1021/bi00706a022. [DOI] [PubMed] [Google Scholar]
- Krishna G., Harwood J. P. Requirement for guanosine triphosphate in the prostaglandin activation of adenylate cyclase of platelet membranes. J Biol Chem. 1972 Apr 10;247(7):2253–2254. [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Leray F., Chambaut A. M., Hanoune J. Role of GTP in epinephrine and glucagon activation of adenyl cyclase of liver plasma membrane. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1385–1391. doi: 10.1016/0006-291x(72)90866-2. [DOI] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Gilman A. G. Adenylate cyclase assay with adenylyl imidodiphosphate and product detection by competitive protein binding. Biochim Biophys Acta. 1974 Jul 17;358(1):154–163. doi: 10.1016/0005-2744(74)90267-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Siegel M. I., Cuatrecasas P. Epinephrine stimulation of fat cell adenylate cyclase: regulation by guanosine-5'-triphosphate and magnesium ion. Mol Cell Endocrinol. 1974 Apr;1(2):89–98. doi: 10.1016/0303-7207(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Aurbach G. D. Binding of 5'-guanylyl-imidodiphosphate to turkey erythrocyte membranes and effects on beta-adrenergic-activated adenylate cyclase. J Biol Chem. 1974 Dec 10;249(23):7630–7636. [PubMed] [Google Scholar]
- Symons R. H. Modified procedure for the synthesis of 32P-labelled ribonucleoside 5'-monophosphates of high specific activity. Biochim Biophys Acta. 1968 Feb 26;155(2):609–610. doi: 10.1016/0005-2787(68)90205-0. [DOI] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Activation of thyroid membrane adenylate cyclase by purine nucleotides. J Biol Chem. 1973 Jan 10;248(1):350–355. [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


