Abstract
Background
Denatonium, a widely used bitter agonist, activates bitter taste receptors on many cell types and plays important roles in chemical release, ciliary beating and smooth muscle relaxation through intracellular Ca2+-dependent pathways. However, the effects of denatonium on the proliferation of airway epithelial cells and on the integrity of cellular components such as mitochondria have not been studied. In this study, we hypothesize that denatonium might induce airway epithelial cell injury by damaging mitochondria.
Methods
Bright-field microscopy, cell counting kit-8 (CCK-8) assay and flow cytometry analysis were used to examine cellular morphology, proliferation and cell cycle, respectively. Transmission electron microscopy (TEM) was used to examine mitochondrial integrity. JC-1 dye and western blotting techniques were used to measure mitochondrial membrane potential and protein expression, respectively.
Results
For airway epithelial cells, we observed that denatonium significantly effects cellular morphology, decreases cell proliferation and reduces the number of cells in S phase in a dose-dependent manner. TEM analysis demonstrated that denatonium causes large amplitude swelling of mitochondria, which was confirmed by the loss of mitochondrial membrane potential, the down-regulation of Bcl-2 protein and the subsequent enhancement of the mitochondrial release of cytochrome c and Smac/DIABLO after denatonium treatment.
Conclusions
In this study, we demonstrated for the first time that denatonium damages mitochondria and thus induces apoptosis in airway epithelial cells.
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-015-0183-9) contains supplementary material, which is available to authorized users.
Keywords: Denatonium, Bitter taste receptors, Epithelium injury, Mitochondria, Cytochrome c
Introduction
Denatonium is a widely used bitter taste agonist and has been demonstrated to activate bitter taste receptors on various cell types including taste cells [1], enteroendocrine cells [2,3], hindbrain neurons [4], airway epithelial cells [5], nasal solitary chemosensory cells [6] and airway smooth muscle cells [7]. Activation of bitter taste receptors increases intracellular Ca2+ levels via a G-protein-coupled receptor (GPCR) cascade involving phospholipase C (PLC)/inositol 1,4,5-trisphosphate (IP3) signaling [4-6]. This increase in intracellular Ca2+ leads to neurotransmitter release and taste detection in bitter taste receptor cells [8,9], insulin secretion by pancreatic islets [2], cholecystokinin (CCK) [10] and glucagon-like peptide-1 [11] release from enteroendocrine cells, CCK release from neurons [4], antimicrobial peptide release from nasal solitary chemosensory cells [6], and acetylcholine release from polymodal urethral chemosensory cells [12]. However, the effects of the bitter agonist denatonium on the cellular components of airway epithelial cells, such as mitochondria, have not been studied.
Mitochondria are important cellular structures that supply cellular energy by generating adenosine triphosphate (ATP). Furthermore, mitochondria act as internal calcium stores through the uptake of intracellular free Ca2+, and this is critical for calcium buffering [13]. When mitochondrial membranes are damaged, mitochondria lose their calcium buffering capability [13], and mitochondria-related proteins, such as cytochrome c and Smac/DIABLO, are released from the mitochondria into the cytosol, resulting in cellular apoptosis [1,14-16]. In contrast, Bcl-2 proteins can prevent apoptosis by blocking the release of cytochrome c from mitochondria [17-20]. Thus, mitochondria play significant roles in the cell cycle, cell growth, and cell death [21].
In this study, we hypothesized that denatonium induces airway epithelial cell injury by damaging mitochondria. We found that denatonium inhibits airway epithelial cell proliferation, reduces the number of cells in S phase and increases cellular apoptosis in a dose-dependent manner via a mitochondrial signaling pathway.
Materials and Methods
Cell culture
Airway epithelial cells (human lung cancer cell line A549 and human bronchial epithelial cell lines 16HBE and BEAS-2B) were cultured in Dulbecco’s modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum and 100 U/ml penicillin/streptomycin. Cells were grown as monolayers in a humidified atmosphere containing 5% CO2 at 37°C. The culture medium was replaced with treatment medium containing the desired concentrations of chemicals every 24 h.
Immunohistochemistry assay
Lung tissues were collected from 8- to 10-week-old wild-type C57BL/6 J mice (Animal Center, Fudan University, Shanghai, China) for the immunohistochemistry assay. The experimental protocol was approved by the Committee of Animal Care of Fudan University. All animals were handled in accordance with the Guideline for the Care and Use of Laboratory Animals. Briefly, lung tissues were harvested and fixed in formalin and then embedded in paraffin. The sections were incubated with primary antibodies including taste receptor type 2 member 10 (TAS2R10), guanine nucleotide-binding protein G(t) subunit alpha-3 (GNAT3), and transient receptor potential cation channel subfamily M member 5 (TRPM5) (Abcam Inc., Cambridge, MA, USA) and, then, were incubated with horse radish peroxidase-linked secondary antibody.
Measurement of intracellular Ca2+ signaling
To monitor free intracellular Ca2+ in epithelial cells, Hank's balanced salt solution (HBSS) with 5 μM Fluo-4/AM (Invitrogen, Carlsbad, CA) was applied to A549 cells for 30 minutes at room temperature. Subsequently, the cells were kept in HBSS for another 20 minutes at room temperature before imaging. Confocal imaging was performed using a Nikon A1R confocal laser scanning microscope system (Nikon Corp., Tokyo, Japan). Fluo-4 was excited with a 488-nm laser, and fluorescence images (512 × 512 pixels) were collected. Regions of interest (ROI; 3 × 3 pixels) were selected in individual epithelial cells using ImageJ software (v. 1.42, Wayne Rasband, NIH) to track the changes in fluorescence intensity. The fluorescence intensity ratio (F/F0) was calculated by dividing fluorescence intensity at time t (F) with the fluorescence intensity at the beginning of the experiment (F0).
Morphological examination of airway epithelial cells
To examine the morphological change in airway epithelial cells, 8 × 105 cells were plated in a 60-mm tissue culture dish and were treated with denatonium benzoate (Sigma-Aldrich, St. Louis, Missouri, USA). As our preliminary results indicated that different airway epithelial cells had differing sensitivities to denatonium, 16HBE, BEAS-2B, and A549 cells were treated with denatonium for 24 h, 48 h, and 72 h, respectively, to achieve similar percentages of cell death for all three cell lines. The bright field images of the airway epithelial cells were taken with a fluorescent microscope (Olympus Corp., Tokyo, Japan) connected to a digital camera. Ten randomly chosen microscopic fields from each dish were analyzed.
Cell proliferation and viability assay
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Japan) was used to assess the rate of cellular proliferation and to quantify cell viability. In brief, airway epithelial cells were plated in 96-well plates at approximately 2000 cells per well with 100 μL culture medium and were treated with denatonium at different concentrations and for different lengths of time. Then, 10 μl of CCK8 solution was applied to each well, and the plates were incubated for 1 h at 37°C. Finally, the absorbance values at 450 nm were determined using a microplate reader (FLX800TBID, Biotek instruments, VT, USA). All experiments were conducted in triplicate.
Transmission Electron Microscopy (TEM) analysis of cellular components
After a 72 h-incubation with 2 mM denatonium, the treated and non-treated control A549 cells were collected, washed with PBS and then fixed in 2.5% glutaraldehyde (PH = 7.4) at 4°C overnight. After washing with 0.1 M phosphoric acid solution, the cells were post-fixed in 1% buffered osmium tetroxide, dehydrated in graded alcohols, embedded in Epon 812, cut into 50–60 nm ultra-thin sections and stained with uranyl acetate and lead citrate. Finally, ultrathin sections were examined with a transmission electron microscope (CM120, Philips, Hillsbro, USA).
Mitochondrial membrane potential measurement
A JC-1 mitochondrial membrane potential assay kit (Beyotime Biotechnology Inc., Nantong, China) was used to detect changes in mitochondrial membrane potential. JC-1 is a fluorescent lipophilic cationic probe. It accumulates in healthy mitochondria to form J-aggregates emitting red fluorescence at 590 nm and accumulates in depolarized mitochondria as J-monomers emitting green fluorescence at 490 nm. An increased ratio of green-fluorescent cells indicates mitochondrial damage. The assay was performed according to the kit’s instructions, and the results of the assay were obtained by flow cytometry (Accuri Cytometers, Inc., MI, USA).
Detecting apoptosis by flow cytometry
An annexin V-FITC and propidium iodide (PI) double staining kit (Invitrogen, Carlsbad, CA, USA) was used to analyze cellular apoptosis. Airway epithelial cells were seeded into 6-well plates (5 × 105 cells/well) and treated with denatonium at different concentrations and for different lengths of time. The cells were digested with trypsin (Gibco® Trypsin-EDTA, Invitrogen, Carlsbad, CA, USA), washed with PBS three times, suspended in 500 μl binding buffer and, finally, incubated with 5 μl of FITC-conjugated Annexin-V and 5 μl of PI for 15 min at room temperature in the dark. Then, the samples were analyzed by flow cytometry.
Cell cycle analysis
Airway epithelial cells were plated in 6-well plates and treated with denatonium at different concentrations and for different lengths of time. The cells were collected, washed with PBS, and fixed in chilled 70% ethanol for 24 h at 4°C. Then the fixed cells were stained with propidium iodide (PI) staining solution (40 μg/ml RNase A and 50 μg/ml PI in PBS) in the dark for 30 min at room temperature. Finally, cell cycle distribution was analyzed by flow cytometry.
Western blot assay
Total protein was extracted using a RIPA kit (Beyotime Biotechnology Inc., Nantong, China). Mitochondrial and cytosolic fractions were separated using a Cell Mitochondria Isolation Kit (Beyotime Biotechnology Inc., Nantong, China). The isolated proteins were separated on polyacrylamide gels and transferred to PVDF membranes. The membranes were incubated with anti-cytochrome c (Cell Signaling Technology (CST), MA, USA), anti-Smac/DIABLO (CST), anti-Cox IV (CST), anti-Bcl-2 (CST), anti-actin (CST), anti-TAS2R4 (Abcam, Cambridge, MA, USA), and anti-GNAT3 (Abcam) at 4°C overnight and were then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobin G at room temperature for 1 hour. The proteins were visualized using Pierce ECL Western Blotting Substrate and autoradiography. The blots were analyzed using Quantity One 4.6 software.
Statistical analysis
The data are expressed as the means ± SEM of at least three independent experiments. The statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. A p-value <0.05 was considered statistically significant.
Results
Expression of bitter taste receptors and their downstream signaling effectors
Immunohistochemistry studies were conducted to investigate the protein expression of bitter taste receptors and their downstream signaling effectors in airway epithelial cells. We found that the bitter taste receptor TAS2R10 and its downstream signaling effectors GNAT3 (guanine nucleotide binding protein, alpha transducing 3) and TRPM5 (transient receptor potential cation channel, subfamily M, member 5) were highly expressed on airway epithelial cells in mice (Figure 1A-C). Furthermore, we also detected the protein expression of the bitter taste receptor TAS2R4 and its downstream signaling effector GNAT3 on A549 cells (Figure 1D). The in vitro experiments showed that 1 mM denatonium triggered Ca2+ oscillations in A549 human epithelial cells (Figure 1E). The Ca2+ oscillations started immediately after denatonium application and lasted for a few cell cycles (Figure 1F).
Figure 1.
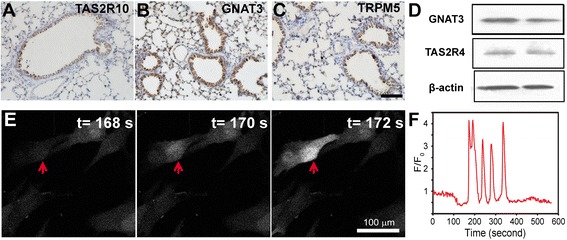
Functional expression of bitter taste receptors and their downstream signaling effectors. A) Immunohistochemistry images showed that bitter taste receptor TAS2R10 and its downstream signaling effectors GNAT3 (B) and TRPM5 (C) were highly expressed on airway epithelial cells in mice. Scale bar: 100 μm. D) Western blot showed that bitter taste receptor TAS2R4 and GNAT3 were expressed on A549 cells. E) In vitro experiments showed that 1 mM denatonium triggered Ca2+ oscillations in A549 human epithelial cells. A549 cells were stained with Fluo-4 to visualize intracellular free Ca2+. Red arrow points to the region of interest (ROI) in an A549 cell. F) Ca2+ oscillations started immediately after denatonium application and lasted for a few cell cycles.
Denatonium inhibits epithelial cell proliferation and increases apoptosis
To determine whether denatonium affects the growth of airway epithelial cells, we measured the proliferation of airway epithelial cells (A549, 16HBE, and BEAS-2B cells) treated with denatonium. Denatonium treatment induces dose-dependent cellular morphology changes. As shown in Figure 2A&B and Additional file 1: Figure S1A, untreated airway epithelial cells are densely packed, whereas airway epithelial cells treated with denatonium were rounded, shrunken, and detached from each other.
Figure 2.
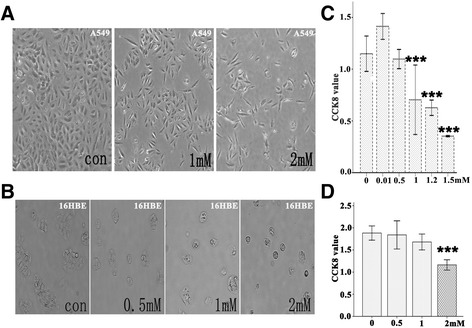
Denatonium inhibits A549 and 16HBE cell proliferation and induces cell morphological changes. A) Bright-field images of cultured A549 cells showed that treatment with denatonium for 72 h induced cell morphological changes. B) Bright-field images of cultured 16HBE cells showed that treatment with denatonium for 24 h induced cellular morphology changes. C) Denatonium markedly inhibited the growth of A549 cells in a dose-dependent manner. D) Denatonium markedly inhibited the growth of 16HBE cells in a dose-dependent manner. One representative experiment with n = 3 is shown. The error bars represent mean values ± SEM. ***indicates significant difference at p < 0.001 versus control.
To further confirm the effects of denatonium on airway epithelial cell proliferation, the CCK-8 assay was used to assess cell proliferation and quantify cell viability. As shown in Figure 2C&D and Additional file 1: Figure S1B, denatonium markedly inhibited the growth of airway epithelial cells in a dose-dependent manner.
Denatonium induces apoptosis of airway epithelial cells and reduces the number of airway epithelial cells in S phase
To evaluate the effects of denatonium on airway epithelial cell apoptosis, we performed an annexin V-FITC/PI double staining assay and flow cytometry analysis. The cells in the upper-right (UR) and lower-right (LR) quadrants of the FACS histogram represent apoptotic cells. As shown in Figure 3 and Additional file 2: Figure S2, denatonium treatment of airway epithelial cells resulted in more apoptotic cells compared with no treatment. We also explored the effect of denatonium on the cell cycle of airway epithelial cells and found that 2 mM denatonium exposure caused a drastic reduction in the number of cells in S phase compared with no treatment (Figure 3 and Additional file 2: Figure S2).
Figure 3.

Flow cytometric analysis of apoptosis induction and cell cycle distribution in A549 and 16HBE cells. A) A549 cells were treated with denatonium (1 mM or 2 mM) for 72 h, stained with FITC-annexin V/PI and then analyzed by flow cytometry. The right panel shows the apoptosis rates of the cells of the various groups. Flow cytometry was also used to analyze DNA at the G1, S, and G2 phases of the cell cycle. B) 16HBE cells were treated with denatonium (0.5 mM, 1 mM or 2 mM) for 24 h. Then the number of apoptotic cells and the number of cells in different stages of the cell cycle were detected by flow cytometry. Data are representative of three similar experiments. **indicates significant difference at p < 0.01 versus control and ***indicates significant difference at p < 0.001 versus control.
Denatonium induces mitochondrial damage
To examine whether denatonium induces mitochondrial damage in airway epithelial cells, we used transmission electron microscopy to examine the ultrastructures of A549 cells treated with denatonium. The control cells appeared to have normal mitochondria and mostly homogeneous cytoplasms (Figure 4A (a1 and a2)), while A549 cells treated with denatonium showed large amplitude swelling of mitochondria (Figure 4A (b1, b2 and b3)) (2 mM for 72 h).
Figure 4.
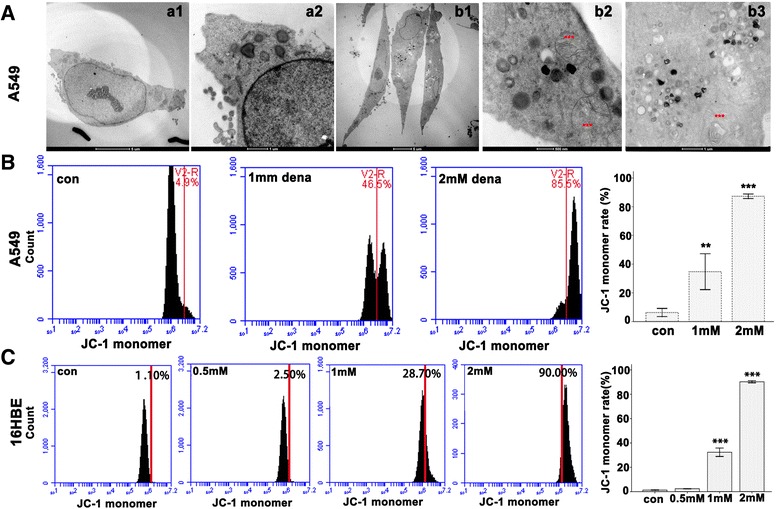
Denatonium reduces mitochondrial membrane potential. A) The TEM microphotographs show the ultrastructure of control cells (a1,a2) and denatonium (2 mM, 72 h) treated A549 cells (b1, b2, b3). Swollen mitochondria (***) were observed in treated cells (b2, b3). B) A549 cells were stained with the cationic lipophilic dye, JC-1 and analyzed by FACS after treatment with denatonium for 72 h. C) 16HBE cells were stained with the cationic lipophilic dye JC-1 and analyzed by FACS after treatment with denatonium for 24 h. The figures are representative profiles of at least three experiments. The histogram shows the quantification of mitochondrial membrane potential. (**p < 0.01, ***p < 0.001).
Denatonium decreases mitochondrial membrane potential
Loss of mitochondrial membrane potential is a sign of the apoptotic process in cells. To further confirm mitochondrial damage, we stained airway epithelial cells with the cationic lipophilic dye JC-1. JC-1 accumulates in healthy mitochondria as aggregates with red fluorescence, while in depolarized or damaged mitochondria, JC-1 transforms into monomers with green fluorescence. As shown in Figure 4 and Additional file 3: Figure S3, denatonium treatment of airway epithelial cells resulted in a dose-dependent increase in the percentage of green-fluorescent-positive cells, which are shown in the right (R) quadrant of the fluorescence activated cell sorter (FACS) histogram. The mitochondrial membrane potential was reduced substantially (drastic increase in JC-1 monomer) in airway epithelial cells when they were treated with 2 mM denatonium.
Denatonium down-regulates the expression of the anti-apoptotic protein Bcl-2 and enhances the release of cytochrome c and Smac/DIABLO from mitochondria in 16HBE cells
To investigate the molecular mechanism underlying denatonium-induced airway epithelial cell apoptosis, we used western blotting techniques to explore the influence of denatonium on the expression of Bcl-2 protein and mitochondrial-related proteins. After treatment with 2 mM denatonium for 24 h, the expression of the anti-apoptotic protein Bcl-2 was significantly reduced (Figure 5A), and the release of cytochrome c and Smac/DIABLO from the mitochondria to the cytoplasm was drastically increased in 16HBE cells (Figure 5B and C).
Figure 5.

Denatonium down-regulates the expression of the anti-apoptotic protein Bcl-2 and enhances the release of cytochrome c and Smac/DIABLO from mitochondria in 16HBE cells. A) The expression of the anti-apoptotic protein Bcl-2 was significantly reduced in 16HBE cells after treatment with denatonium for 24 h. B) In 16HBE cells treated with denatonium for 24 h, the cytochrome c and Smac/DIABLO levels in mitochondria were significantly reduced, whereas, the cytochrome c and Smac/DIABLO levels in cytosol were significantly increased (C). The figures are representative profiles of at least three experiments. (***p < 0.001).
Discussion
Denatonium, one of the most bitter known substances, has been widely used as a bitter taste agonist and has many functions in various organs. In this study, we found that denatonium inhibits airway epithelial cell proliferation, reduces the number of cells in S phase and increases cell apoptosis in a dose-dependent manner via a mitochondrial signaling pathway. For the first time, we demonstrated that denatonium decreases mitochondrial membrane potential and induces mitochondrial damage in airway epithelial cells.
For decades, millimolar doses of denatonium have been used in most studies. For example, 1–2.5 mM denatonium has been commonly used to induce bitter taste [1,22,23], to relax smooth muscle cells [7,15], and to study the beating of cilia on airway epithelial cells [5]. In this study, we tested the effects of 0.5-2 mM denatonium on A549, 16HBE, and BEAS-2B cells and found that denatonium induced apoptosis in all three of these airway epithelial cell lines. The three cell lines had different sensitivities to denatonium, Approximately 30% apoptosis was observed in A549 cells after 2 mM denatonium exposure for 72 h (Figure 3A), in 16HBE cells after 1 mM denatonium exposure for 24 h (Figure 3B), and in BEAS-2B cells after 2 mM denatonium exposure for 48 h (Additional file 2: Figure S2A).
Mitochondria play critical roles in multiple cellular functions including 1) generation of ATP to supply cellular energy, 2) storage of intracellular free Ca2+, and 3) release of apoptotic factors, such as cytochrome c and Smac/DIABLO, which initiate nuclear death [17-20]. In this study, we found that denatonium caused mitochondrial damage as indicated by mitochondrial swelling and the loss of mitochondrial membrane potential after denatonium treatment. The loss of mitochondrial membrane potential opens the mitochondrial permeability transition pores resulting in the release of cytochrome c and Smac/DIABLO from the mitochondria. Furthermore, we found that denatonium down-regulated Bcl-2 protein expression, which has been demonstrated to prevent the release of cytochrome c from the mitochondria [17-20]. Decreased mitochondrial function is one of the key events in apoptotic death. Thus, it is not surprising that denatonium induces the inhibition of airway epithelial cell proliferation, increases apoptotic cell death, and reduces the number of cells in S phase of the cell cycle.
In recent years, bitter taste receptors have been found to be expressed in an increasing number of organ systems and to mediate a variety of functions [2-12]. However, it is challenging to study the bitter taste receptors and their downstream signaling pathways for several reasons. First, the human genome contains ~25 TAS2R genes, several members of which are expressed on human cells to detect bitter compounds [5]; thus, it is difficult to block all these receptors using molecular techniques, such as RNA interference. Secondly, bitter agonists can activate at least four cellular mechanisms that increase intracellular Ca2+ levels, including 1) the TAS2R/β,γ-gustducin/PLCβ2/IP3 pathway [4-6]; 2) the TAS2R/α-gustducin/cyclic nucleotide monophosphate pathway [16]; 3) direct potassium channel inhibition [14]; and 4) direct nonselective cation channel activation [20]. Thus, it is also difficult to block all the downstream signaling pathways of bitter agonists. Thirdly, although several studies have reported inhibitory effects of probenecid [17] and amino acid derivatives [19] on bitter taste receptors, no widely tested bitter taste receptor antagonists are available. Thus, our current strategy to study the bitter taste receptors and their downstream signaling pathways was to use multiple bitter taste agonists, such as denatonium, chloroquine, saccharin, colchicine, quinine, salicin, strychnine and yohimbine. However, almost every bitter agonist commonly used has side effects: for example, chloroquine is an antimalarial, a potent autophagic drug, that inhibits Na+,K+-ATPase activity [18]. As denatonium [1,2,12,22-28] and chloroquine [29] have both been used as the sole bitter agonist in many studies, denatonium- and chloroquine-induced effects as bitter taste receptor activation should be interpreted with caution.
Conclusions
For the first time, we demonstrated that denatonium inhibits airway epithelial cell proliferation, reduces the number of cells in S phase and increases cell apoptosis in a dose-dependent manner via a mitochondrial signaling pathway. Denatonium plays critical roles in taste detection, neurotransmitter release, smooth muscle relaxation, and innate immunity, and could be potentially used for asthma treatment; however, the underlying mechanism of action of denatonium is unknown. We studied the effects of denatonium on mitochondria and revealed a novel mitochondrial-related signaling pathway.
Acknowledgement
This project was supported by grants from the Natural Science Foundation of China (81400018, 81170013 and 81400002), from the Doctoral Program of Higher Education of China (20130071120063), and from Shanghai Committee of Science and Technology (12411950101).
Abbreviations
- DMEM
Dulbecco’s modified Eagle medium
- TAS2R4
Taste receptor type 2 member 4
- TAS2R10
Taste receptor type 2 member 10
- GNAT3
Guanine nucleotide-binding protein G(t) subunit alpha-3
- TRPM5
Transient receptor potential cation channel subfamily M member 5
- CCK-8
Cell Counting Kit-8
- TEM
Transmission Electron Microscopy
Additional files
Denatonium inhibits BEAS-2B cell proliferation and induces cell morphological changes. A) Bright-field images of cultured BEAS-2B cells showed that treatment with denatonium for 48 h induced cell morphological changes. One representative experiment with n = 3 is shown. B) Denatonium markedly inhibited the growth of BEAS-2B cells in a dose-dependent manner at 48 h. The error bars represent mean values ± SEM. *** indicates significant difference at p < 0.001 versus control.
Flow cytometric analysis of apoptosis induction and cell cycle distribution in BEAS-2B cells. A) BEAS-2B cells were treated with denatonium (0.5 mM, 1 mM or 2 mM) for 48 h, stained with FITC-annexin V/PI and then analyzed by flow cytometry. The right panel shows the apoptosis rates of the cells in the various groups. B) Flow cytometry was used to analyze DNA in cells in the G1, S, and G2 phases of the cell cycle. Data are representative of three similar experiments.(**P < 0.01, ***P < 0.001).
Denatonium reduces mitochondrial membrane potential. A) We stained BEAS-2B cells with the cationic lipophilic dye JC-1 and analyzed them by FACS. The figures are representative profiles of at least three experiments. B) The histogram shows the quantification of mitochondrial membrane potential. (**P < 0.01 and ***P < 0.001).
Footnotes
Xiaoxing Wen and Jian Zhou contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CB and HL supervised the conduction of the entire project. XW and JZ performed the experiments, analyzed the data and drafted the manuscript. DZ, JL, QW, NF, HZ, YS performed some of the experiments. All authors read and approved the final manuscript.
Contributor Information
Xiaoxing Wen, Email: wenxiaoxing666@163.com.
Jian Zhou, Email: zhou.jian@fudan.edu.cn.
Dan Zhang, Email: zhangdan6250@yeah.net.
Jing Li, Email: liumiao.lijing@163.com.
Qin Wang, Email: wangqin19851125@126.com.
Nana Feng, Email: fnn2005508@126.com.
Haixing Zhu, Email: starfishzhu@gmail.com.
Yuanlin Song, Email: song.yuanlin@zs-hospital.sh.cn.
Huayin Li, Email: li.huayin@zs-hospital.sh.cn.
Chunxue Bai, Email: bai.chunxue@zs-hospital.sh.cn.
References
- 1.Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–5. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- 2.Straub SG, Mulvaney-Musa J, Yajima H, Weiland GA, Sharp GW. Stimulation of insulin secretion by denatonium, one of the most bitter-tasting substances known. Diabetes. 2003;52:356–64. doi: 10.2337/diabetes.52.2.356. [DOI] [PubMed] [Google Scholar]
- 3.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci. 2002;99:2392–7. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008;294:R33–8. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 5.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–50. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- 9.Spielman AI, Huque T, Nagai H, Whitney G, Brand JG. Generation of inositol phosphates in bitter taste transduction. Physiol Behav. 1994;56:1149–55. doi: 10.1016/0031-9384(94)90359-X. [DOI] [PubMed] [Google Scholar]
- 10.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–39. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 11.Kim K-S, Egan JM, Jang H-J. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. Diabetologia. 2014;57:2117–25. doi: 10.1007/s00125-014-3326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci. 2014;111:8287–92. doi: 10.1073/pnas.1402436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of basal calcium levels in mouse taste cells. J Neurophysiol. 2008;100:2177–91. doi: 10.1152/jn.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akabas M, Dodd J, Al-Awqati Q. Identification of electrophysiologically distinct subpopulations of rat taste cells. J Membr Biol. 1990;114:71–8. doi: 10.1007/BF01869386. [DOI] [PubMed] [Google Scholar]
- 15.Pulkkinen V, Manson ML, Säfholm J, Adner M, Dahlén S-E. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol. 2012;303:L956–66. doi: 10.1152/ajplung.00205.2012. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, et al. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. 1995. [DOI] [PubMed]
- 17.Greene TA, Alarcon S, Thomas A, Berdougo E, Doranz BJ, Breslin PA, et al. Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS One. 2011;6:e20123. doi: 10.1371/journal.pone.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang B, Meng N, Zhao B, Zhao J, Zhang Y, Zhang S, et al. Protective effects of a synthesized butyrolactone derivative against chloroquine-induced autophagic vesicle accumulation and the disturbance of mitochondrial membrane potential and Na+, K + −ATPase activity in vascular endothelial cells. Chem Res Toxicol. 2009;22:471–5. doi: 10.1021/tx8002824. [DOI] [PubMed] [Google Scholar]
- 19.Pydi SP, Sobotkiewicz T, Billakanti R, Bhullar RP, Loewen MC, Chelikani P. Amino acid derivatives as bitter taste receptor (T2R) blockers. J Biol Chem. 2014;289:25054–66. doi: 10.1074/jbc.M114.576975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsunenari T, Hayashi Y, Orita M, Kurahashi T, Kaneko A, Mori T. A quinine-activated cationic conductance in vertebrate taste receptor cells. J Gen Physiol. 1996;108:515–23. doi: 10.1085/jgp.108.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Ogura T, Kinnamon SC. IP3-Independent release of Ca2+ from intracellular stores: a novel mechanism for transduction of bitter stimuli. J Neurophysiol. 1999;82:2657–66. doi: 10.1152/jn.1999.82.5.2657. [DOI] [PubMed] [Google Scholar]
- 23.Ogura T, Mackay-Sim A, Kinnamon SC. Bitter Taste Transduction of Denatonium in the MudpuppyNecturus maculosus. J Neurosci. 1997;17:3580–7. doi: 10.1523/JNEUROSCI.17-10-03580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clapp TR, Trubey KR, Vandenbeuch A, Stone LM, Margolskee RF, Chaudhari N, et al. Tonic activity of Gα-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–7. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang PM, Verma A, Bredt DS, Snyder SH. Localization of phosphatidylinositol signaling components in rat taste cells: role in bitter taste transduction. Proc Natl Acad Sci. 1990;87:7395–9. doi: 10.1073/pnas.87.19.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund TC, Kobs AJ, Kramer A, Nyquist M, Kuroki MT, Osborn J, et al. Bone marrow stromal and vascular smooth muscle cells have chemosensory capacity via bitter taste receptor expression. PLoS One. 2013;8:e58945. doi: 10.1371/journal.pone.0058945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawano S, Seto E, Mori T, Hayashi Y. G-protein-dependent and -independent pathways in denatonium signal transduction. Biosci Biotechnol Biochem. 2005;69:1643–51. doi: 10.1271/bbb.69.1643. [DOI] [PubMed] [Google Scholar]
- 28.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci. 2010;107:3210–5. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C-H, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11:e1001501. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]


