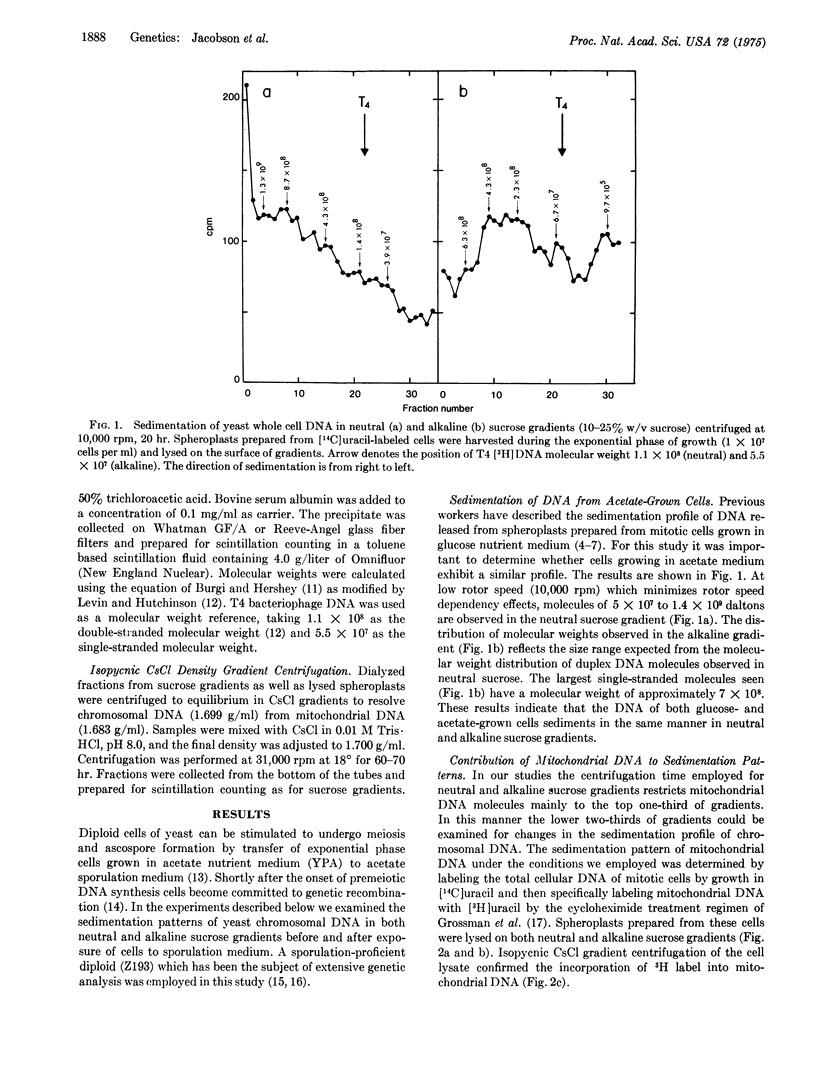
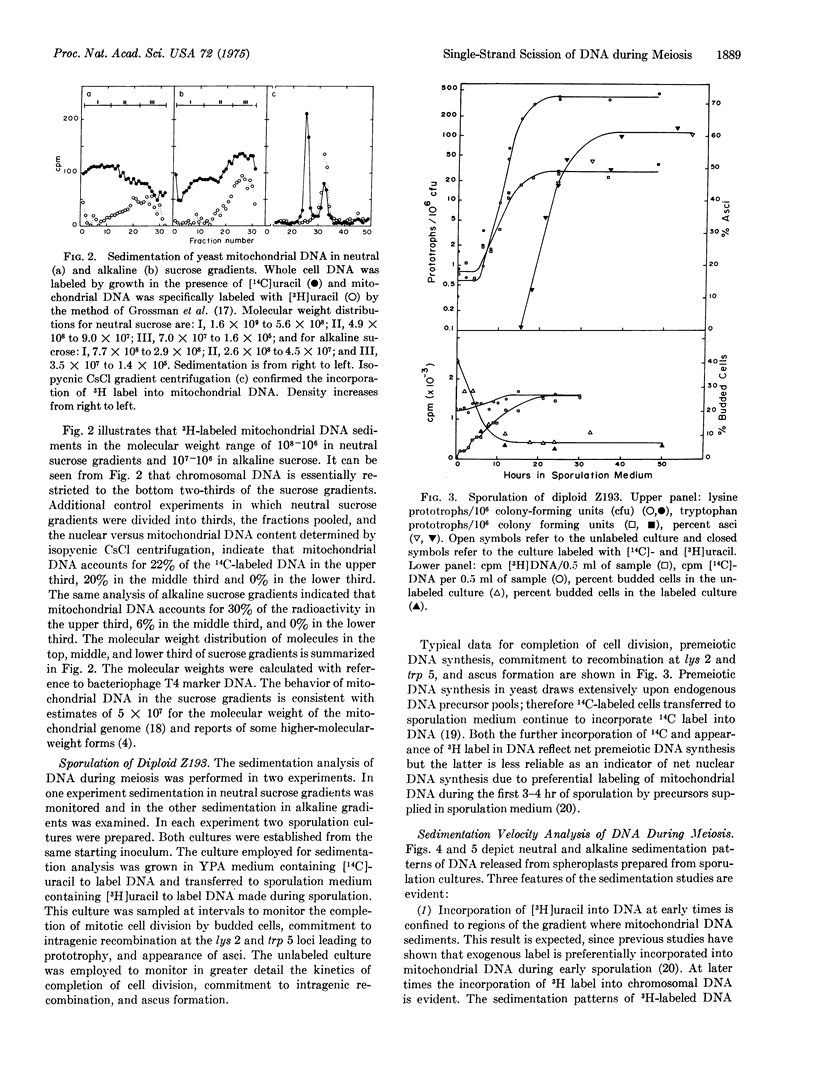
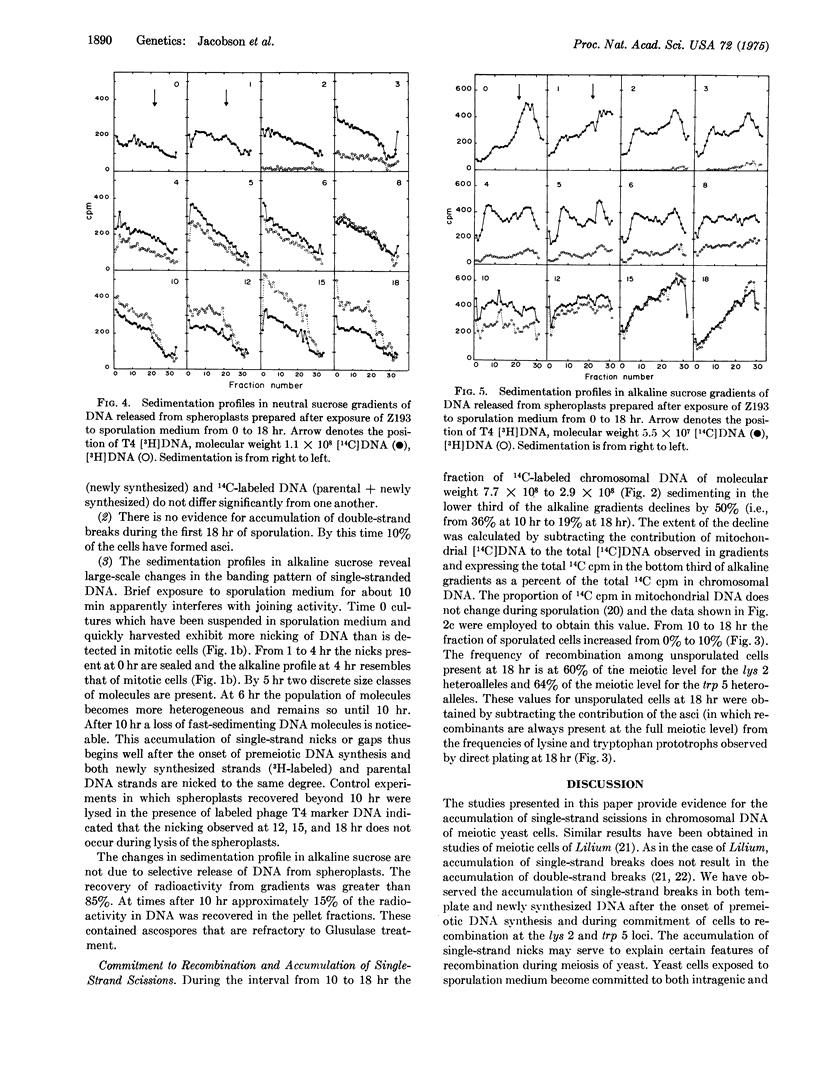
Abstract
Diploid cells of the yeast Saccharomyces cerevisiae induced to undergo meiosis accumulate single-strand scissions in both template and newly synthesized DNA during commitment to genetic recombination. No evidence for accumulation of double-strand breaks during meiosis was obtained. When commitment to recombination is at the full meiotic level there are approximately 70 to 200 single-strand scissions per meiotic cell in which approximately 150 recombination events have been reported to occur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bicknell J. N., Douglas H. C. Nucleic acid homologies among species of Saccharomyces. J Bacteriol. 1970 Feb;101(2):505–512. doi: 10.1128/jb.101.2.505-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamire J., Cryer D. R., Finkelstein D. B., Marmur J. Sedimentation properties of yeast nuclear and mitochondrial DNA. J Mol Biol. 1972 Jun 14;67(1):11–24. doi: 10.1016/0022-2836(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. Genes controlling meiosis and spore formation in yeast. Genetics. 1974 Sep;78(1):215–225. doi: 10.1093/genetics/78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. The genetic control of sporulation in Saccharomyces. I. The isolation of temperature-sensitive sporulation-deficient mutants. Genetics. 1969 Jan;61(1):79–89. doi: 10.1093/genetics/61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Esposito M. S. Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3172–3176. doi: 10.1073/pnas.71.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J Bacteriol. 1973 Nov;116(2):925–930. doi: 10.1128/jb.116.2.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Grossman L. I., Goldring E. S., Marmur J. Preferential synthesis of yeast mitochondrial DNA in the absence of protein synthesis. J Mol Biol. 1969 Dec 28;46(3):367–376. doi: 10.1016/0022-2836(69)90182-x. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Holliday R. Molecular aspects of genetic exchange and gene conversion. Genetics. 1974 Sep;78(1):273–287. doi: 10.1093/genetics/78.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Molecular basis for genetic recombination. Genetics. 1974 Sep;78(1):247–257. doi: 10.1093/genetics/78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst D. D., Fogel S., Mortimer R. K. Conversion-associated recombination in yeast (hybrids-meiosis-tetrads-marker loci-models). Proc Natl Acad Sci U S A. 1972 Jan;69(1):101–105. doi: 10.1073/pnas.69.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Hutchinson F. Neutral sucrose sedimentation of very large DNA from Bacillus subtilis. I. Effect of random double-strand breaks and centrifuge speed on sedimentation. J Mol Biol. 1973 Apr 15;75(3):455–478. doi: 10.1016/0022-2836(73)90454-3. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Esposito R. E., Esposito M. S. Aberrant nuclear behavior at meiosis and anucleate spore formation by sporulation-deficient (SPO) mutants of Saccharomyces cerevisiae. Exp Cell Res. 1974 Jan;83(1):166–174. doi: 10.1016/0014-4827(74)90700-9. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Sedimentation properties of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1188–1191. doi: 10.1073/pnas.69.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Newlon C. S., Byers B., Fangman W. L. Yeast chromosomal DNA: size, structure, and replication. Cold Spring Harb Symp Quant Biol. 1974;38:9–16. doi: 10.1101/sqb.1974.038.01.004. [DOI] [PubMed] [Google Scholar]
- Piñon R., Salts Y., Simchen G. Nuclear and mitochondrial DNA synthesis during yeast sporulation. Exp Cell Res. 1974 Feb;83(2):231–238. doi: 10.1016/0014-4827(74)90334-6. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Lopez E., Zamb T. J., Roth R. Role of premeiotic replication in gene conversion. Nature. 1975 Jan 17;253(5488):212–214. doi: 10.1038/253212a0. [DOI] [PubMed] [Google Scholar]
- Simchen G., Piñon R., Salts Y. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res. 1972 Nov;75(1):207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]


